
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Fill in the blanks please
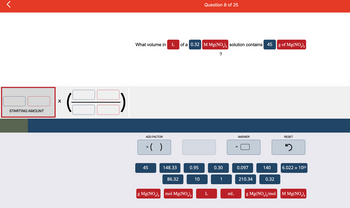
Transcribed Image Text:<
STARTING AMOUNT
X
What volume in
ADD FACTOR
x( )
45
g Mg(NO3)₂
L
of a 0.32 M Mg(NO₂)₂ solution contains 45
?
148.33
86.32
0.95
mol Mg(NO3)₂
Question 8 of 25
10
L
0.30
1
mL
ANSWER
0.097
210.34
140
0.32
g Mg(NO₂)₂/mol
g of Mg(NO3)₂
RESET
3
6.022 x 1023
M Mg(NO₂)₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chemical properties include (choose the best answer) Melting and boiling point Density Reactivity with other chemicals and molecules Solubility in various solventsarrow_forwardPlease help.arrow_forwardce the following equations: _103+ HSO3 + H* + _HSO3 → -√₂ + + _H₂O 103 ->> CamScanner _H* + -1₂ + — SO-2 H₂O 94² + SOA H*arrow_forward
- The following drawings represent the same molecules. H Н. H True False Harrow_forwardBaCl2٠2H2O What is the mass salt? What is the mass water? What is the mass percent?arrow_forwardView History Bookmarks Develop Window Help A east.cengagenow.com [References) 1 pt EXERCISE Unit Conversion 1 pt Drag conversion units onto the boxes in the equation to make conversions. Some boxes can be left empty. Click on a unit to remove it from its position. 1 pt 1 cm³ Cu 9 g Cu 9.5 x 1021 atoms Cu 1 g Cu 1 pt 1 kg 1000 g 1 cm = 1 mL 1 pt 1 L 1000 cm3 1 pt 1 pt 520 L x 1 pt 1 pt 1 pt A piece of copper has a volume 520 L. What is the mass of the sample, in units of grams? In the boxes above, enter the correct setup that would be used to solve this problem. 1 pt 1 pt Check Next (2 of 3) 1 pt 1 pt Submit Answer Try Another Version 2 item attempts remaining 1 ptarrow_forward
- The periodic law states that elements in the same _____________ will have similar physical and chemical characteristicsarrow_forwardI am struggling to get this question right and would really appreciate any help. Pls make sure you’re anser is corrrecf 100% pls and thank you !arrow_forwardmistry F20 SE 1. If you have 23.2 grams of gold, what is the volume of the sample? Densities of Some Common Metals Metal Density (g/mL) Copper 8.94 the Aluminum 2.70 Gold 19.3 (A) 0.116 ml (B) 0.832 mL (C) 1.20 ml (D) 8.59 mL DELLarrow_forward
- plz explain the answer choicesarrow_forwardPercentage Abundance. The element rubidium consists of two isotopes, one having an isotopic mass of 84.912 and the other having an isotopic mass of 86.920. Determine the percentage abundance of the lighter isotope. Record the answer to the correct number of significant figures. Show the numerical set-ups. Include units and the correct number of significant figures in the set-up.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY