
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
a)
b)

Transcribed Image Text:What type(s) of intermolecular forces are expected between CH3CH,NH2 molecules?
H
-C
·C
N-
H
Indicate with a Y (yes) or an N (no) which apply.
dipole forces
induced dipole forces
hydrogen bonding
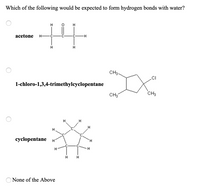
Transcribed Image Text:Which of the following would be expected to form hydrogen bonds with water?
acetone
H-
CH3-
1-chloro-1,3,4-trimethylcyclopentane
CH3
CH3
H
cyclopentane
H.
H.
H
H.
None of the Above
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What constitutes a 'reference material', and why does its utilization play a criticalrole in the chemical analysis of food products? Provide examples.arrow_forwardStandard Reduction (Electrode) Potentials at 25 Half-Cell Reaction * (volts) F2(8) + 2 e2F (aq) 287 Ce* (aq) +e Ce (aq) 1.61 Mn0, (aq) + BH'(aq) +5e Mn"( "(aq) + 4 H20(1) 1.51 Cla(g) +2e -2 C (aq) 1.36 Cr20, (aq) + 14 H'(aq) - 6e2 Cr*"(aq) + 7 H20() 1.33 Oz() + 4 H'(aq) + 4e2 H20(1) 1.229 Br20) + 2 e2 Br (aq) 1.08 NO3 (aq) + 4 H'(aq) + 3eNO(g) + 2 H20() Hg"(aq) 0.96 2 Hg (aq) + 2e 0.920 Hg"(aq) + 2 e- Hg(1) 0.855 Ag (aq) +e Ag(s) 0.799 Hg2" (aq) - 2e-2 Hg(1) 0.789 Fe" (aq) +e Fe"(ag) 0.771 12(s) + 2e 21 (aq) 0.535 Fe(CN)"(aq) + e Fe(CN), (aq) 0.48 Cu (aq) + 2eCu(s) 0.337 Cu"(aq) +e- Cu (aq) 0.153 S(s) + 2 H'(aq) + 2 e H2S(aq) 0.14 2H'(aq) + 2e-H2(8) 0.0000 Pb (aq) + 2e- Ph(s) -0126 Sn (aq) + 2 e Sn(s) -014 Ni (aq) + 2 e- Ni(s) -0.25 Co (aq) + 2 e Co(s) -0.28 "(aq) + 2e Cd(s) -0.403 Cr"(aq) +e "(aq) -041 Fe"(aq) + 2 e Fe(s) -044 Cr"(aq) +3e-Cr(s) -0.74 Zn"(aq) +2 e- Zn(s) -0.763 2 H20() +2e Hz(z) + 2 OH (aq) -0.83 Mn"(aq) + 2e - Mn(s) -1.18 A" (aq) + 3e- Al(s) -1.66 Mg (aq) + 2 e- Mg(s)…arrow_forwardlim (sin x · ln x) = x→0+ e 2 8.arrow_forward
- The process whereby chemical treatment is used to temporarily prevent decomposition is most completely defined as: Disinfection Restoration Public health measures Preservationarrow_forwardExpress (5.25*10^4) megameters to metersarrow_forwardAssuming that you weighted a piece of silver five times and each time you got 52 grams. However, the actual weight from the Bureau of Standards said it should weigh 50 g. (a) What is the % relative errors? (b) What type of error does this exemplify? (c) Does this affect (a) reliability (b) Accuracy (c) both a & barrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY