
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
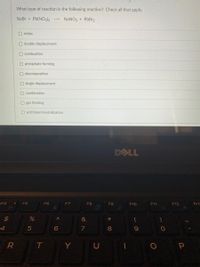
Transcribed Image Text:What type of reaction is the following reaction? Check all that apply.
NaBr + Pb(NO3)2 --> NANO3 + PbBr2
O redox
O double displacement
O combustion
O precipitate forming
O decomposition
O single displacement
O combination
O gas forming
O acid base/neutralization
DELL
F4
F5
F6
FZ
F8
F9
F10
F11
F12
Prt
%24
&
4
7
8
9.
R
T
Y U
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In a 1.0 x 10-6 M solution of Ba(OH)₂ (aq) at 25 °C, arrange the species by their relative molar amounts in solution. Ba(OH)2 H₂O Ba + H₂O* Greatest amount Least amount Answer Bank OH™arrow_forwardYou are performing an experiment in lab that involves the titration of 25.0 mL of H2SO4 solution. You titrate the acidic solution with 0.8067 M NaOH and the equivalence point is reached by the addition of 17.31 mL of NaOH solution. Using the balanced equation below, calculate the molartity of H2SO4 in the flask. Do NOT include units. 2NaOH(aq) + H2SO4(aq) --> 2H2O(l) + Na2SO4(aq)arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- Pls explained the whole steps with correct solution i will upvote you otherwise downvote remember downvotearrow_forwardWhen the following equation is balanced properly under basic conditions, what are the coefficients of the species shown? |520,²- + 1 so,2- I, 2 I +1 Water appears in the balanced equation as a product (reactant, product, neither) with a coefficient of 3 (Enter O for neither.) How many electrons are transferred in this reaction?4arrow_forward2E. Sodium dihydrogen phosphate (first proton only) and sodium hydroxide Please helparrow_forward
- Write the molecular equation which completes the following. The reaction occurs in an aqueous solution. K2S(ag) + HCl(ag) - (Use the lowest possible coefficlents. Be sure to specify states such as (aq) or (s). If a box is not needed, leave It blank. Use H* for hydronium lon.) +arrow_forward. 2- ( 2 Write the basic equilibrium equation for HS. Be sure to include the proper phases for all species within the reaction. e + 2 (s) H3O+ for additional resources 3 OH ( Reset 4 Question 8 of 26 (1) S LO 6 • x H₂O H 7 (g) → 8 H₂O 9 11 (aq)arrow_forwardIf reacting 50 mL of 0.100 M NaOH with 50 mL 0.100 HCI resulted in a AHxn= - 50 kJ what would be the new AH if 50 mL of 0.200 M NaOH were reacted with 50 mL 0.200 HCI? a) + 25 kJ b) - 50 kJ c) + 100 kJ d) - 25 kJ e) + 50 kJarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY