
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
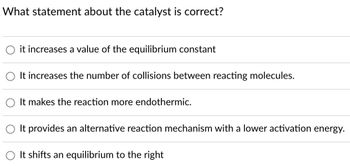
Transcribed Image Text:What statement about the catalyst is correct?
it increases a value of the equilibrium constant
It increases the number of collisions between reacting molecules.
It makes the reaction more endothermic.
It provides an alternative reaction mechanism with a lower activation energy.
It shifts an equilibrium to the right
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4) Which of the following statements is true of a catalyst? A) Its concentration stays constant throughout the reaction C) it provides a new pathway for the reaction B) it increases the rate of a reaction D) all of the abovearrow_forwardMatch the Term with the definition 1. Equilibrium position 2. Chemical equilibrium 3. Reaction quotient 4. Law of mass action 5. Equilibrium constant C a. used to determine if a reaction has reached equilibrium b. depends on the initial concentrations of the substances in a reaction c. states that every reaction proceeds to an equilibrium state with a specific Keq d. the point at which the reaction appears to "stop" e. the ratio of product concentration to reactant concentration at equilibrium 6. For the reaction N2 (g) + O2(g) 2 NO(g) that is run at 25 °C, Kc = 1.2 × 10-30. Use this information to calculate the equilibrium co for the reaction 2 NO(g) N2 (g) + O2(g) a. 8.3 x 1029 b. 1.2 x 10-30 C. 1.7 x 1030 d. 1.1 x 10-15 e. 6.0 × 10-31 7. Write the equilibrium expression for the formation of nitrosyl bromide. a. [NO] 2 [Br]/[NOBr] 2 b. [NOBr]2/[NO] 2 c. [NOBr] 2/[NO] 2 [Br2] d. [NO] 2[Br2] / [NOBr] 2 e. [NOBr] 2/[NO]² 2NO(g) + Br2 (g) 2NOBr(g)arrow_forwardWhich among the following statements is/are FALSE about the collision theory? I. The rate of the reaction increases with an increasing number of effective collisions. II. The reacting species can collide in any orientation to become bonded with each other. III. Any amount of energy is required during a collision to allow formation of new bonds. Which among the following statements is/are FALSE about the collision theory? I. The rate of the reaction increases with an increasing number of effective collisions. II. The reacting species can collide in any orientation to become bonded with each other. III. Any amount of energy is required during a collision to allow formation of new bonds. All of the above I and III only I and II only II and III onlyarrow_forward
- At equilibrium, the forward rate is faster than the reverse rate. True O Falsearrow_forwardA catalyst increases the activation energy required for a reaction to proceed. True Falsearrow_forwardwhen an equilibrium is disrupted (“stressed”) due to changes that a chemist imposes on the system, Le Chatelier’s Principle predicts that the equilibrium will shift in the direction that will undo as much of the change as possible. For instance, if a particular reactant compound is removed through some chemical means, then the equilibrium will shift to favor the reactants, so as to replace some of the lost compound. In this lab, you will observe the effects of stress on the tetrachlorocobaltate(II)/hexaquocobalt(II) equilibrium shown below: CoCl42-(solv) + 6 H2O(solv) Co(OH2)62+(solv) + 4 Cl-(solv) blue pink The (solv) means a solvated species. In this system, ethanol is the solvent. This means that water can not be excluded from the reaction quotient any longer, since it is now a solute in ethanol and therefore has a measurable concentration. The color of the solution provides a visual clue about the dominant cobalt species…arrow_forward
- The catalyst influences the rate of reaction in which ways? nothing happens O Decreases it O Increases it O Stops the reactionarrow_forwardWhich of the following is not true of catalysts? O They become part of the final product. O They are unchanged by the reaction they catalyze. O They speed up reactions. O They do not change the normal position of a chemical equilibrium.arrow_forwardHow does a catalyst influence the rate of a reaction?arrow_forward
- Which is the appropriate description to show the effect of a catalyst on the reaction rate and equilibrium in a reversible reaction: Reactants › Products O• The rate of the forward reaction is increased. • The rate of the reverse reaction is decreased 0 •The equilibrium position is displaced to the right O • The rate of the forward reaction is increased. The rate of the reverse reaction is increased The equilibrium position is unchanged. • The rate of the forward reaction is increased. 0 •The rate of the reverse reaction is unchanged • The equilibrium position is displaced to the right • The rate of the forward reaction is unchanged. The rate of the reverse reaction is unchanged The equilibrium position is unchanged. Suppose that an exothermic reaction, Reactants < › Products, is at equilibrium. According to Le Chatelier's Principle, if the reaction temperature is increased, in which direction will the equilibrium be displaced? O The equilibrium will be displaced toward the…arrow_forwardIncreasing the concentration of the reactants will slow down the reaction. O true O falsearrow_forwardA catalyst increases the activation energy of a reaction and thus facilitates the reaction True Falsearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY