
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
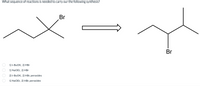
Transcribed Image Text:What sequence of reactions is needed to carry our the following synthesis?
Br
Br
1) t-BUOK, 2) HBr
1) NaOEt, 2) HBr
2) t-BUOK, 2) HBr, peroxides
1) NaOEt, 2) HBr, peroxides
OO O O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the suitable reagents for the given reaction and arrange them accordingly. OH A+&+F OEt 1 2 OEt OEt 1) EtOH, H₂SO4; 2) EtOH, H₂SO4 1) EtONa followed by H₂O; 2) TsCl/py followed by EtONa 1) EtONa followed by H₂O; 2) NaH followed by EtBr O 1) EtOH, H₂SO4; 2) NaH followed by EtBr O 1) EtOH, H₂SO4; 2) TsCmy followed by EtONaarrow_forwardHh.118.arrow_forwardWhich reagents would be best to achieve the following synthesis? Br A) 1) t-BuOK 2) BH₂-THF 3) H₂O2, NaOH B) 1) NaOEt 2) BH3-THF 3) H₂O2, NaOH LOH C) 1) NaOEt 2) Hg(OAc)2, H₂0 3) NaBH4 D) 1) 1-BuOK 2) Hg(OAc)2, H₂O 3) NaBH4arrow_forward
- Which of the following reagents will result in the following reaction occurring? O H+/H₂O/Heat H₂O OH-/H₂O/Heat LIAIH4 O ZI O OH + H3Narrow_forwardOChem question regarding acidity Consider ONLY the hydrogen drawn in each compound, list the compounds in order of INCREASING acidity. Also, explain why the most acidic is considered the most acidic and why the least acidic is considered least acidic. Thank you!arrow_forwardWhich reactions have a positive ASXN? 2 A(g) + 3 B(g) 4C(g) O 2 A(g) + B(g) C(g) 2 A(g) + B(g) 4C(g) 2 A(g) + B(s) 3 C(g) 全arrow_forward
- The starting material that completes the following reaction is: 1) NH3 2) H₂/Ni ? Draw Your Solution NH2arrow_forwardO O U Which reagents would be best to achieve the following synthesis? B Ph Br A) 1) t-BuOK 2) H₂O+ B) 1) t-BuOK 2) BH₂-THF 3) H₂O2, NaOH C) 1) BrMg 2) H3O+ OH Ph OH D) 1) Mg 2) H 3) H3O+ E) 1) Mg 2) Å 3) H3O+arrow_forwardBe sure to answer all parts. Draw a stepwise mechanism for the following reaction: HBr HO. Br Part 1 HOBR Br HBr vlow etructure view structure но Part 2: H,0 HBr HOBR но view structure view atructure Br Part 3 vlew atructure vlew atructure Part 4 out of 4 Br finish atructure 平 1 一 1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY