
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
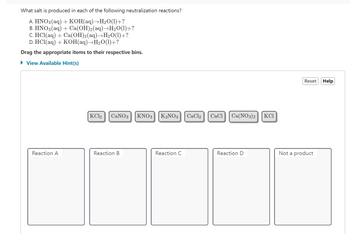
Transcribed Image Text:What salt is produced in each of the following neutralization reactions?
A. HNO3(aq) + KOH(aq)→H₂O(1)+?
B. HNO3(aq) + Ca(OH)2(aq)→H₂O(1)+?
C. HCl(aq) + Ca(OH)2(aq)→H₂O(1)+?
D. HCl(aq) + KOH(aq)→H₂O(1)+?
Drag the appropriate items to their respective bins.
► View Available Hint(s)
Reaction A
KC12 CaNO3
Reaction B
KNO3 K₂NO3 CaCl2
Reaction C
CaCl Ca(NO3)2
Reaction D
KCI
Reset
Not a product
Help
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The chemical equation below, FeCl3(aq) + 3NaOH(aq) → Fe(OH)3(s) + 3 NaCl(aq) is an example of what type of reaction? A.) acid-base B.) combustion C.) oxidation-reduction D.) precipitationarrow_forwardHow many grams of nitric acid, HNO₃, are required to neutralize (completely react with) 4.30 g of Ca(OH)₂ according to the acid-base reaction: 2 HNO₃(aq) +Ca(OH)₂(aq)→2 H₂O(l)+Ca(NO₃)₂(aq)arrow_forwardcomplete and balance the following molecular equations, and then write the net ionic equation for each. a. HBr9aq) + Ca9OH)2 b. Cu(OH)2 (s) + HClO4 (aq) c. Al(OH)3 (s) = HNO3(aq)arrow_forward
- Which of the following is the net ionic equation for: CaBr2 (aq) + Na₂CO3 (aq) → ... Make sure you create a balanced equation and use the solubility chart to determine solubility. O Ca²+ (aq) + 2Br (aq) + 2 Nat (aq) + CO32 (aq) → 2 Na+ (aq) + 2 Br (aq) + CaCO3 (s) O CaBr2 (aq) + Na2CO3 (aq) → 2 NaBr (aq) + CaCO3 (s) O Ca2+ (aq) + CO32- (aq) → CaCO3 (s) O Br₂ (aq) + 2 Na+ (aq) → 2 NaBr (aq) O 2 Br (aq) + 2 Na* (aq) → 2 NaBr (aq)arrow_forwardComplete the reactions and balance the reactions Double displacement Reactions: 1. 2LİCI(aq) + Pb(NO3)2(aq) > 2. Н:СО3(аq) 3. N2OH(aq) + NHẠCI(aq) > 4. HaSOa(aq) + Ca(ОН)2(аq) > 5. Potassium Sulfate (aq) + Calcium chloride (aq) →arrow_forward4. H₂S (aq) + Reaction type: 5. CaCO3 (s)→ CO₂ (g) + Reaction type: 6. C4H10O (g) + O₂(g) → Reaction type: 7. Ss (s) + Reaction type: 8. H₂SO4 (aq) + Reaction type: AsCl3 (aq) →__ AS2S3 (s) + Fe (s) → 9. H3PO4 (aq) + Reaction type: Al (s) → CaO (s) CO₂ (g) + FeS (s) _Al2(SO4)3 (aq) + NH4OH (aq) → HCI (aq) H₂O (e) - H₂(g) (NH4)3PO4 (aq) + HOH (e)arrow_forward
- b. 3H2SO4(aq) + 2Al(OH);(aq) → Al2(SO4)3(aq) + 6H2O(1) 4. In each of the following, identify the reactant that is oxidized and the reactant that is red a. 2Li(s) + F2(g) → 2LİF(s) b. Cl2(g) + 2KI(aq) → I2(s) + 2KCI(aq) atoms of H 5. Calculate each of the following a. number of Li atoms in 4.5 moles ofLi. b. moles of Cu in 7.8 x 10²1 atoms of Cu. c. moles of C2H6 in 3.75 x 1023 molecules of C2H6.arrow_forward4. Calculate the calculate the mass of precipitate formed from 5.0 g of strontium chloride. Li3PO4(aq) → Sr3(PO4)2(S) + LiCl(aq) _SrCl₂(aq) + 5. What mass of aluminum hydroxide in needed to neutralize 5.00 g of hydrochloric acid? Al(OH)3(s) + _HCl(aq) → ___AlCl3(aq) + _H₂O(1) 195arrow_forwardFf.141.arrow_forward
- Choose balanced molecular, ionic, and net ionic equations for the reaction of hydrochloric acid with strontium and gallium. (Both are oxidized by hydrogen ions.) 1. Molecular equation for the reaction of hydrochloric acid with strontium a. Sr (s) + 2HCl(aq) → Sr Cl2(aq) + H2(g) b. Sr (s) + 2HCl(aq) → Sr Cl2(aq) + H2(g) + H2O c. Sr (s) + 2H+(aq) → Sr 2+(aq) + H2(g) d. Sr (s) + 2H+(aq) + 2Cl–(aq) → Sr 2+(aq) + 2Cl–(aq) + H2(g) 2. Ionic equation for the reaction of hydrochloric acid with strontium a. Sr (s) + 2HCl(aq) → Sr Cl2(aq) + H2(g) b. Sr (s) + 2H+(aq) → Sr 2+(aq) + H2(g) c. Sr (s) + 2H+(aq) + 2Cl–(aq) → Sr 2+(aq) + 2Cl–(aq) + H2(g) d. Sr (s) + 2HCl(aq) → Sr Cl2(aq) + H2(g) + H2O 3. Net ionic equations for the reaction of hydrochloric acid with strontium a. Sr (s) + 2H+(aq) → Sr 2+(aq) + H2(g) b. Sr (s) + 2HCl(aq) → Sr Cl2(aq) + H2(g) c. Sr (s) + 2H+(aq) + 2Cl–(aq) → Sr 2+(aq) + 2Cl–(aq) + H2(g) d. Sr (s) + 2HCl(aq) → Sr Cl2(aq) + H2(g) + H2O 4.…arrow_forwardStrong acid-base reactions are a subcategory of double displacement reactions. The hydrogen ion of the acid "switches places" with the cation of the base, while the hydroxide ion of the base "switches places" with the anion of the acid. 1. Write the correct product(s) of the following reaction. Include physical states! Also balance the chemical reaction by writing correct coefficients. NaOH(aq) + HCl(aq) 2. Write the correct product(s) of the following reaction. Include physical states! Also balance the chemical reaction by writing correct coefficients. _NaOH(aq) + H SO (aq) 3. Suppose it takes 10 mL of 0.1 M HCl to neutralize 10 mL of 0.1 M NAOH. (“M" = molarity, which is a unit of concentration.) How many milliliters of 0.1 M H,SO, do you expect will be needed to neutralize 10 mL of 0.1 M NaOH? Explain your choice.arrow_forwardF2 (g) + Ga(s) → Ga (aq) + F (aq) a. F2 (g) + Ga(s) Ga+ (ag) + F(aq) b. O2 (9) + H2O(1) +Pb(s) → Pb(OH)2 (s) O2(g) + H2O(1) + Pb(s) |Pb(OH)2(s) 3+ H* (aq) + MnO4 (ag) + Fe (ag) → Mn²+ (aq) + Fe* (ag) + H2O(1) с. H (aq) + MnO4 (aq) + Fe?+ (aq) Mn2+ (aq) + Fe+ (aq) + H2O(1)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY