
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
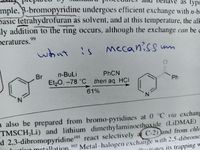
Transcribed Image Text:mple. B-bromopyridine undergoes efficient exchange with n-b
basic tetrahydrofuran as solvent, and at this temperature, the alk
ly addition to the ring occurs, although the exchange can be c
as typi
peratures.9
what
mecaniss mm
Br
n-Buli
PHCN
Et,0, -78 °C then aq HCI
Ph
61%
N.
n also be prepared from bromo-pyridines at 0 °C via exchang
TMSCH,Li) and lithium dimethylaminoethoxide (LIDMAE)
react selectively a C-2) and from chla
Metal-halogen exchange with 2,5-dibrome
d 2,3-dibromopyridine
uiuo metallation, 102
Ilurtrates its trapping w
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. A 0.36 M hydrocyanic acid (HCN) solution has a pH of 4.87. Give: (a) the balanced equation for the ionization of hydrocyanic acid; (b) K. of HCN; (c) % ionization of HCN. a. b.arrow_forward2. Let's prepare an H₂SO4 solution by dissolving 4.9 grams of H₂SO a. How man b. is the molarity of the solution? is the pH of the solution? C. Wr H₂SO4 molecules are in this solution? d. Wir her is datin ފނމފރ ހރތ **S in 100 mL of water. pH of the solution if adding 3.0 grams of NaOH to the solution?arrow_forwardThe hydronium ion concentration in an aqueous solution at 25°C is 7.7x10-2 M. |M. The hydroxide ion concentration is | The pH of this solution i | The pOH isarrow_forward
- 98 grams of sodium acetate (Mol. wt =82 g/mol) were placed in 764 mL of water such that the final volume was 784 mL. What is the pH of the solution?arrow_forward8:43 1 ll 5GE Question 11 of 25 Submit The pH for 0.0715 M solution of CCl,CO2H is 1.40. Determine the value of Ka for CCl,CO2H. 1 2 Based on the given values, fill in the ICE table to determine concentrations of all reactants and products. CCI,CO,H(+ H20(1) =H;0*(aq) +CCl,CO2 (ac Initial (M) Change (M) Equilibrium (M) 5 RESET 0.0715 1.40 -1.40 0.040 -0.040 0.032 -0.032 +x -X 0.0715 + x 0.0715 - 1.40 + x 1.40 - x 0.040 + x 0.0340 - x 0.032 + x 0.032 - xarrow_forwardWhat mass of HNO, is present in 245.0 mlL of a nitric acid solution having a pH of 490? Mass 3D Jmgarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY