
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Question 12 of 60
Submit
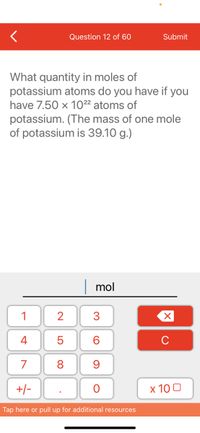
What quantity in moles of
potassium atoms do you have if you
have 7.50 x 10²² atoms of
potassium. (The mass of one mole
of potassium is 39.10 g.)
| mol
1
3
4
6.
C
7
8
+/-
х 100
Tap here or pull up for additional resources
2.
LO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the mass in grams of 4.78 × 1021 atoms of chromium. (The mass of one mole of chromium is 52.00 g.)arrow_forwardA scientist is trying to discover information about an unknown metal in a compound. The formula for the compound is believed to be XBr, where X is the unknown metal. The scientist determined that a 4.720 g sample of this compound contains 4.745 x 10-2 mol Br. Calculate the atomic mass of the unknown metal, X. atomic mass = amu What is the identity of the metal? Provide the name or symbol of the element. metal:arrow_forwardDetermine the mass in grams of 3.11 × 10²¹ atoms of arsenic. (The mass of one mole of arsenic is 74.92 g.)arrow_forward
- what quantity in moles of potassium do you have if you have 5.70 x 10^22 atoms of potassium. ( the mass of one mole of potassium is 39.10 g)arrow_forwardDetermine the mass in grams of 7.94 × 10²¹ atoms of zinc. (The mass of one mole of zinc is 65.39 g.)arrow_forwardWhat is the mass of 7.10 moles of magnesium chloride, MgCl2? Express your answer with the appropriate units. How many moles of nitrogen, N, are in 77.0 g of nitrous oxide, N2O? Express your answer with the appropriate units.arrow_forward
- how many moles of sodium atoms do you have if you have 7.10x10^23 atoms of sodium. (The mass of one mole of sodium is 22.99 g)arrow_forwardDetermine the mass in grams of 3.92 × 10²¹ atoms of arsenic. (The mass of one mole of arsenic is 74.92 g.)arrow_forwardDetermine the mass in grams of 6.65 × 10²¹ atoms of barium. (The mass of one mole of barium is 137.33 g.)arrow_forward
- Determine the mass in grams of 4.65 × 1221 atoms of barium. (The mass of one mole of barium is 137.33g.)arrow_forwardDetermine the mass in grams of 4.54 × 10²¹ atoms of zinc. (The mass of one mole of zinc is 65.39 g.arrow_forwardDetermine the mass in grams of 5.30 × 10²¹ atoms of chromium. (The mass of one mole of chromium is 52.00 g.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY