
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:M Your Chemis X b Answered: × b Similar Que x
с
←
<
app.101edu.co
STARTING AMOUNT
+
X
Aktiv Chemi X
2.143
ADD FACTOR
2.02
mol H₂
x( )
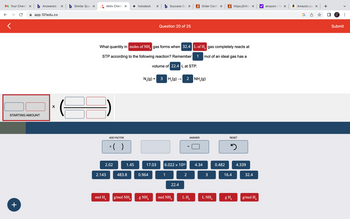
What quantity in moles of NH, gas forms when 32.4 L of H₂ gas completely reacts at
3
2
STP according to the following reaction? Remember 1
mol of an ideal gas has a
1.45
483.8
holodeck × |
g/mol NH₂
3
17.03
0.964
Success Co X
Question 20 of 25
g NH
volume of 22.4 L at STP.
N₂(g) + 3 H₂(g) → 2 NH₂(g)
6.022 x 10²3
1
22.4
mol NHg
2
=
Order Confi x
ANSWER
LH₂
2
4.34
3
LNH,
0.482
https://inform X
RESET
3
16.4
g H₂
4.339
32.4
g/mol H₂
y! amazon - Ya X
a Amazon.com x +
G
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 530 mmHg to atmarrow_forwardHow many moles of sodium are needed to produce 17.7 L of hydrogen gas according to the following reaction at 0 °C and 1 atm? sodium(s) + water (l) → sodium hydroxide (aq) + hydrogen (g) Amount = molesarrow_forwardPart A) Fill out the following table Part B)You have a 1.50 L weather balloon at 35.5 oC and 0.850 atm of pressure. The balloon floats across the country. When it lands it is 1.50 L in volume at 1.10 atm. Assume no gas escaped from the balloon what is the new temperature? Part C) Draw the products of the reaction NaOH + HClO4 --> ? Part D) You need to give a sheep medicine to prevent heartworms. the medicine is 0.08% (m/v) solution of ivermectin. The sheep need 0.024 g of ivermectin, how many mL of medicine is this?arrow_forward
- Determine the volume of O2 (at STP) formed when 50.0 g of KClO3 decomposes according to the following reaction. The molar mass for KClO3 is 122.55 g/mol.2 KClO3(s) → 2 KCl(s) + 3 O2(g)arrow_forwardHow many grams of gas are present in a 5.6 liter (STP) sample of SO2 gas? 1 mole gas = 22.4 L at STParrow_forwardWhen 25.0 g of potassium chlorate (KCIO3) decomposes, how many L of O2 gas are formed at STP? Refer to the chemical equation below. 2 KCIO3(s) - 2 KCl(s) + 3 O2(g) 012.4 L 02 024.8 L 02 06.85 L 02arrow_forward
- What are the partial pressures of the gases? If the gas was consumed completely, put 0 for the answer. atm PNO atm Po, Incorrect atm PNO, Incorrectarrow_forward8. A gas mixture contains Ar, He, and Kr at partial pressures of 125, 175, and 225 mmHg, respectively. If Ne is added until the total pressure is 623 mmHg, what is the partial pressure of Ne?arrow_forward15. A fuel-air mixture contains 0.5 mol CH4(g), 1.5 mol 02(g), and 6.0 mol N2(g), under a total pressure Ptot = 25 atm. Find the partial pressure of 02(g) in it: A) 25 atm B) 0.063 atm C) 1.6 atm D) 19 atm E) 4.7 atmarrow_forward
- 2.- A sample of hydrogen gas initially at .85 atm is cooled from 10°C to -40°C at a constant volume. What is its final pressure (in atm)? 0.4544 .6998 5.5 1.1 OOO0arrow_forwardHow many moles of NH₃ form when 32.4 L of H₂ gas completely reacts at STP according to the following reaction? Remember 1 mol of an ideal gas has a volume of 22.4 L at STP N₂(g) + 3 H₂(g) → 2 NH₃(g)arrow_forwardAn ideal gas “follows all the rules,” what causes gas to “break the rules?”arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY