
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
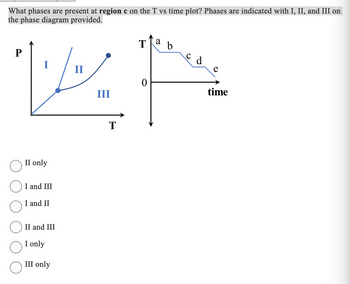
Transcribed Image Text:What phases are present at region c on the T vs time plot? Phases are indicated with I, II, and III on
the phase diagram provided.
منانا
P
II only
I and III
I and II
II and III
I only
III only
II
III
T
T a b
0
d
e
time
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ll.68.arrow_forwardA simplifed phase diagram for iron is shown below, with the solid part divided into the body-centred cubic (BCC) and face-centred cubic (FCC) phases The line dividing the BCC and FCC forms in almost, but not quite vertical. Predict which way this line slopes and explains.arrow_forwardThe vapor pressure of an unknown solid is given by ln(P/Torr)=22.413−(2211/T), and the vapor pressure of the liquid phase of the same substance is approximately given by ln(P/Torr)=18.352−(1736/T). Determine the enthalpy of vaporization, the enthalpy of sublimation, and the enthalpy of fusion. Then calculate the triple point temperature and pressurearrow_forward
- CaCO3(s)⟶CaO(s)+CO2(g)CaCO3(s)⟶CaO(s)+CO2(g) Express your answer as a chemical equation. Identify all of the phases in your answer. Keep the order of compounds as in the original equation.arrow_forwardThe figures below are of temperature monitos with blue fluid in which a tube (that was also been filled with the blue fluid has been inverted and placed in the beaker (open on the end that's submerged in the blue fluid). As the temperature is raised on the beaker/tube system that level of blue fluid in the tube will transition A completely full B partially full, C at the same level of blue fluid in the beaker. 20 °C 30° C 55 °C A B C When does the temperature of the fluid represent it's boiling point?arrow_forwardDetermine the vapor pressure of a liquid in kPa at 383 K. The enthalpy change is 29 kJ/mol. At 310 K, its vapor pressure is 19 kPa. Use this information to estimate the pressure of the vapor at the first temperature. Hint: You must put the enthalpy value into J/mol to cancel units with R. Round your answer so that it has 1 decimal place. R= 8.314 J/(mol*K) Your Answer:arrow_forward
- h phase based on w x b Classify each phase based on wh x 0472 Ch 10 HW - Activities and Due Dates> Ch 10 HW Resources GUp! A certain mass of nitrogen gas occupies a volume of 9.22L at a pressure of 7.12 atm. At what pressure will the volume of this sample be 11.60 L? Assume constant temperature and ideal behavior. atm about us privacy policy terms of use contact us help careersarrow_forwardThermodynamics and enthalpy Data: M(Pb) = 207 g mol" AmeltH°(Pb) = 4.8 kJ·mol" AvapH°(Pb) = 179.5 kJ·mol" Cp (Pb, liquid) = 32.4 J mol"' K-' Tmelt = 327°C Tvap = 1749°C Cp (Pb, solid) = 23.6 J mol·' K'' Let's consider the conversion of lead (Pb) in the gas phase at 1749 °C into lead in the solid state at 250 °C under the standard pressure P° = 1 bar. a. Calculate the standard molar enthalpy for this reaction.arrow_forwardAt 307 K, the vapor pressure of a liquid is 15 kPa, and at 389 K, it’s vapor pressure is 121 kPa. Use this information to estimate the enthalpy of vaporization in kJ. R= 8.314 J/(mol*K) (Please type answer)arrow_forward
- Given the phase diagram for iodine (I2) below, predict what happens when an iodine sample is heated from 50°C to 250°C at a constant pressure of 1 atm. Note that the pressure and temperature values are just estimates.arrow_forwardUsing the phase diagram of carbon dioxide, describe the following conditions as stated below. 1. What happens when the follovving changes are made in a CO2 sample, which initially records at 1 atm. and -60°C? (a) Pressure increases at a constant temperature to 80 atm. (b) Temperature increases from -60°C to -18°C at constant 80 atm.pressure.arrow_forwardUsing the heating curve of WATER to answer the following questions, if point 5 represents 100 °C liquid water 1. Identify the region of the graph where the particles are moving with the smallest average speed 2 2. Identify the physical state of point 6 Liquid + Gas 3. What is occurring in region 2 -> 3 in terms of physical change Melting 4. In which region does the substance have a definite shape and definite volume 1. 5. What's the name of the process 5- 6 in terms of physical change Vaporization 6. How is energy changing during 3 -> 5 7. How is energy changing from 7 -> 6 Condensation 3. (50 amaad ua1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY