
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
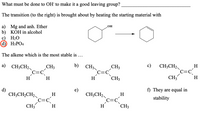
Transcribed Image Text:What must be done to OH´ to make it a good leaving group?
The transition (to the right) is brought about by heating the starting material with
a) Mg and anh. Ether
b) KOH in alcohol
H2O
d) H3PO4
OH
The alkene which is the most stable is ...
a) CH3CH2
b)
с)
CH3CH2
CH3
CH3
CH3
H
H
H
H
CH3
CH3
H
d)
e)
f) They are equal in
CH;CH,CH2
c=c
CH3
CH;CH2
c=c
CH3
H
H
stability
H
H
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
The transition (to the right) is brought about by heating the starting material with a) Mg and anh. Ether b) KOH in alcohol c) H2O d) H3PO4
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
The transition (to the right) is brought about by heating the starting material with a) Mg and anh. Ether b) KOH in alcohol c) H2O d) H3PO4
Solution
by Bartleby Expert
Knowledge Booster
Similar questions
- Predict reagents needed to complete this SN1 reaction. OH A B C D H3O+ HBr HBr heat |- H₂Oarrow_forwardDraw the product of the following reaction. CH3 CH3CHCH2CH2CO₂CH3 1. DIBAH 2. H3O+ • Draw only the product derived from the acyl portion of the carboxylic acid or acid derivative. • You do not have to consider stereochemistry. + ?arrow_forwardIndicate the product or reagents as appropriate (Note: draw the appropriate configuration when necessary)arrow_forward
- Complete the synthesis below by drawing the structure of the organic product in each step. Draw the structure of A, B and C.arrow_forwardCHM 2 1 25 LABORATORY MANUAL VII. Draw the three intermediates, including resonance structures, for the attack of nitronium ion on pyridine. Which position(s) is favored? Why? VH. Draw the two intermediates, including resonance structures, for the attack of nitronium ion on pyrfole. Which position is favored? Why?arrow_forwardWhich O atom of 4-pyrone do you think is more basic?Explain.arrow_forward
- 10. Consider the below reaction between the acetylide ion and methanol. Draw the conjugate acid that is formed as a product in this reaction.arrow_forwardDraw all intermediates and show all electron - pushing OH HBr A. - Br B. 오이 CH3NH2 C. H₂O® E. NaOEt HOEt S D. OHarrow_forward1. You have an alkyl halide and the product has a thiol group without an alkyl, what is the reagant? 2. What is the reaction of disodium sulfide and Na2S, what is its product? 3. You have a thiol compound that reacts with sodium hydroxide, what do you get? 4. You have an oxyrane reacting with sulfuirc acid and methanol, what do you get? 5. Have an alkene reacting with Br, what do you get?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY