
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
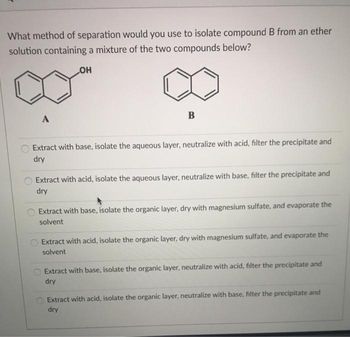
Transcribed Image Text:What method of separation would you use to isolate compound B from an ether
solution containing a mixture of the two compounds below?
OH
A
B
Extract with base, isolate the aqueous layer, neutralize with acid, filter the precipitate and
dry
Extract with acid, isolate the aqueous layer, neutralize with base, filter the precipitate and
dry
Extract with base, isolate the organic layer, dry with magnesium sulfate, and evaporate the
solvent
Extract with acid, isolate the organic layer, dry with magnesium sulfate, and evaporate the
solvent
Extract with base, isolate the organic layer, neutralize with acid, filter the precipitate and
dry
Extract with acid, isolate the organic layer, neutralize with base, filter the precipitate and
dry
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Understanding of food analysis methods. Principles of method described compared to other techniques. 1 The MOHR Titration, AOAC Method? What is it and how does it work? How can it be used to quantify salt in a sample. 2 Are there any advantages and/or disadvantages of this method?3 Are there any alternate methods better than the Mohr Titration toquantitatively determine salt in a sample? 4. Diseases with recent statistics? Refer to World Health Organisation? 5. Why analayse salt? 6. Consumption? 7. Main aims of study?arrow_forwardA mixture of 5 mL of acetone (boiling point 56 ° C) and toluene (boiling point 111 ° C) was separated by fractional distillation. At the end of the distillation, 2.5 mL of the fraction 1 and 1.6 mL of fraction 2. The percent recovery of toluene is: a.39% b.50% C.32% d.82%arrow_forwardWhen a mixture of ethyl butyrate and methyl acetate separated by simple & fractional distillation. How do two methods of separation produce different temperature results?arrow_forward
- Which of these is NOT true of a good recrystallization solvent? Which of these is NOT true of a good recrystallization solvent? a) The compound is insoluble in the solvent when it is cold. b) The solvent boiling temperature is at least 40 °C. c) The solvent has many impurities. d) The compound is soluble in the solvent when it is hot.arrow_forwardStart with 15mL of 0.100M HCl. Pipette 10mL of the 0.100M HCl into a 250mL beaker. Then dilute it to around 100mL with distilled water. What would the concentration of HCl be?arrow_forwardThe following test on compounds X and Y were done and the results, list the observations in the table below. Complete the inferences for each test.arrow_forward
- What is the following and what are the organic molecules? H H H C C Harrow_forwardA tea bag containing 15 grams of tea leaves with 0.7% of caffeine was used in solid-liquid extraction with 200ml of distilled water followed by liquid-liquid extraction using DCM. If 50.0mg of caffeine requires 10 ml of water and 3.0ml of DCM to be dissolved, how much caffeine can be extracted using 70.0ml of DCM? a. 4.8 x 10^-2 b. 5.7 x 10^-2 c. 3.8 x 10^-3 d. 5.8 x 10^-3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY