
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Could I have help with the two following homework problems in chemistry I am not understanding

Transcribed Image Text:Many home barbeques are fueled with propane gas
(C3H8)
Read Sections 7.3, 7.6. You can click on the Review link to access the section in your eText.
Part A
What mass of carbon dioxide (in kg) is produced upon the complete combustion of 16.0 L of propane (approximate contents of
two 5-gallon tanks)? Assume that the density of the liquid propane in the tank is 0.621 g/mL. (Hint: Begin by writing a balanced
equation for the combustion reaction.)
Express your answer in kilograms to three significant figures
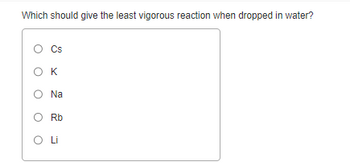
Transcribed Image Text:Which should give the least vigorous reaction when dropped in water?
Cs
OK
Na
Rb
O Li
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A dose of aspirin of 5.0 mg per kilogram of body weight has been prescribed to reduce fever in an infant weighing 8.5 pounds. The number of milligrams of aspirin that should be administered is 19 mg 5.0 mg 0.59 mg 53 mg 1.6 mgarrow_forwardhelp me solve this pleasearrow_forwardHello, good evening. I am currently in Chemistry 1032. I am looking for some help in how to solve this problem. *******The density of whole human blood in a healthy individual is 1.04 g/mL. Given that the density of water at 25 °C is 1.00g/mL, what is the specific gravity of whole human blood?arrow_forward
- What are your worst fears about Chemistry?arrow_forwardAn intramuscular medication is gicen 5.0 mg/kg of body weight. What is the dose for a 180-lb patient?arrow_forwardA patient provides a urine sample. The density of the patient's urine is 1.0212 g/mL. What is the specific gravity of the urine? specific gravity:arrow_forward
- Give “Drug X” 5.0 mg/kg per day in two divided doses. The patient weighs 44 lb.arrow_forwardCholesterol levels are measured in mg/dL in the USA. If your HDL is 150 mg/dL, what is this in ppm? (Assume the density of blood = 1 g/mL) A B C D 150 ppm 0.15 ppm 1500 ppm 15000 ppmarrow_forwardWhat amperage is necessary to plate out 24.5 grams of aluminum onto an object in 12 hours and 35 minutes? Group of answer choices 7.04 A 5.80 A 4220 A 6.02 A 6.20 Aarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY