
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
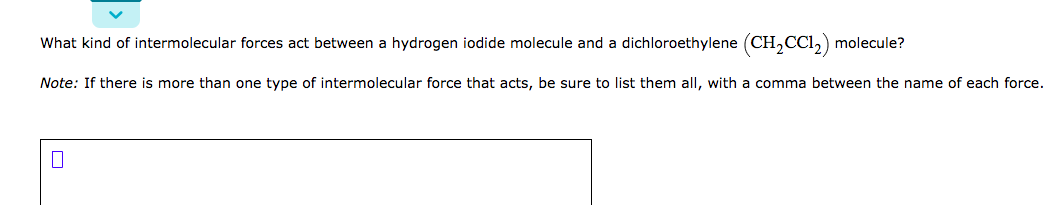
Transcribed Image Text:What kind of intermolecular forces act between a hydrogen iodide molecule and a dichloroethylene (CH,CCI,) molecule?
Note: If there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- What kind of intermolecular forces act between a carbon monoxide molecule and a formaldehyde (H₂CO) molecule? Note: If there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.arrow_forwardWhat kind of intermolecular forces act between a neon atom and a carbon dioxide molecule? Note: If there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force. 0arrow_forwardWhen water boils what forces are overcome? A Intermolecular forces (IMFs) B Covalent bonds с Both IMFs and covalent bondsarrow_forward
- When does a substance exists in a condensed phase? a. When its molecules have enough average kinetic energy to overcome intermolecular forces of attraction. b. When its molecules have too little average kinetic energy to overcome intermolecular forces of repulsion. c. When its molecules have too little average kinetic energy to overcome intermolecular forces of attraction.arrow_forwardDifluoroethene has two isomers with very different properties despite having the same chemical formula. Based on the chemical structure shown for the first isomer, what intermolecular forces are present in this molecule?arrow_forwardWhat kind of intermolecular forces act between a chloromethane (CH3C1) molecule and a hydrogen sulfide molecule? Note: If there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.arrow_forward
- Which of the following intermolecular forces hold ethanol together? ion-dipole forces H-bonding, dispersion, and dipole-dipole forces dispersion forces H-bonding onlyarrow_forwardWhich molecule ( CH3OH or CH4 ) has a greater vapor pressure and why?arrow_forwardWhat kind of intermolecular forces act between a dichloroethylene (CH₂CCl₂) molecule and a hydrogen iodide molecule? Note: If there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force. Xarrow_forward
- Which of the following would exhibit hydrogen bonding in a pure substance? A.)HCl B.)CH3CH3 C.)CF2H2 D.)CH3CH2OH E.)CH3OCH3arrow_forwardLiquid water is more viscus than, say gasoline. And liquid water has a far higher boiling point. Even though each gasoline molecule is far heaver. Describe why this is on a molecular level.arrow_forwardPleasearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY