
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
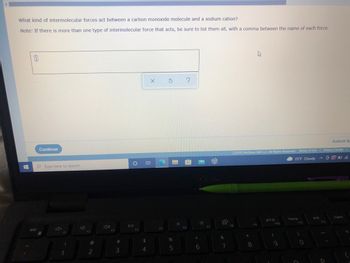
Transcribed Image Text:What kind of intermolecular forces act between a carbon monoxide molecule and a sodium cation?
Note: If there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
?
Submit A
2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |
75°F Cloudy ADEO
home
end
insert
prt sc
1
A
esc
Continue
Type here to search
!
1
F2
2
F3
#
3
F4
Et
$
4
F5
%
5
30%
F6
A
3X
6
F7
8¹
&
7
FB
*
8
F9
F10
(
9
F11
1
0
F12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please don't provide handwritten solution...arrow_forwardPlease I would like you to type the answer instead of writing. Thank youarrow_forwardDrug: ClaritinDraw your molecule (or print out the line structure) on a separate page. 2) For each central atom in your molecule, decide if it is polar or nonpolar. 3) For those central atoms that are polar, a. Color the central atom RED if it forms hydrogen bonds b. Color the central atom GREEN if it forms dipole-dipole interactions 4) Make a reasonable prediction stating whether your molecule will be soluble in water or not soluble and explain your reasoning. If your molecule seems to be borderline in polarity, making it difficult to predict solubility, explain your reasoning and the difficulty involved. 5) Find a source (Pubchem, Merck Index, PDR, RxList etc.) that discusses the water solubility of your drug. Compare your predictions with the information you find on the water-solubility of your drug and comment on any discrepancies.arrow_forward
- Take some water with a straw and put a few drops on plastic sheet. move one of the drops close to another one with your straw. Put a small amount of one of the solids (salt, pepper, sugar, talcum powder) on one of the drops. 1. Does the shape change? 2. Try this again with the other solids.arrow_forwarda) What term best describes the reason why the paperclip is able to “float” on the surface of the water? b) Explain why the paperclip “floats” and doesn’t sink to the bottom of the glass. Connect this explanation to the term you provide in part a. Your explanation must include a discussion of the relevant chemical bonds and their role in this scenario.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY