
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
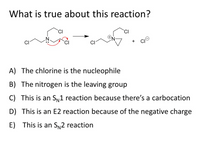
Transcribed Image Text:What is true about this reaction?
`CI
CI
CI
CI
CI
+
A) The chlorine is the nucleophile
B) The nitrogen is the leaving group
C) This is an Sy1 reaction because there's a carbocation
D) This is an E2 reaction because of the negative charge
E) This is an SN2 reaction
Expert Solution
arrow_forward
Step 1
In the given reaction, nitrogen is a nucleophile.
Nitrogen attacks at the carbon atom and release Cl atom as leaving group. This is a single step reaction.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Br Mg What is the oxidative addition step? MgBr 1) O=C:O 2) H₂O* CO₂Harrow_forwardwhat is wrong with the following reaction?arrow_forwardBe sure to answer all parts. Draw the product of the Lewis acid-base reaction. Label the electrophile and nucleophile. CH,CH,OH + BF, window open nucleophile v electrophilearrow_forward
- This image is the final product that is produced. If a synthesis started with benzene and other organic compounds (four carbons or less) what would the synthesis look like to achieve this final product.arrow_forwardDraw the structure of the product of the reaction.arrow_forwardName the product for the following reactionarrow_forward
- 4a. Which type of molecule is this? Label the nonpolar and polar regions of this molecule. Label the ester bonds and phosophodiester bonds. H₂C-0-C HC-0-C CH, CH,−N O CH₂ O CH3 4b. What products would you obtain if you performed an hydrolysis reaction? Draw them. O=C O=0arrow_forwardsolve this reaction and show the productarrow_forwardWhat explains why many aldehydes and ketones can undergo self- condensation reactions in basic conditions? A. The alpha carbon can lose a proton and act like a nucleophile and the carbonyl carbon a an electrophile B. The alpha carbon can gain a proton and act like an electrophile and the carbonyl carbon is a nucleophile C. The oxygen of the carbonyl group can attack the carbon of the carbonyl group D. Only esters can undergo self-condensation reactionsarrow_forward
- Look at the atom in each molecule and classify as an electrophile or a nucleophile. How do you know if it is a electrophile or nucleophile? What makes it an electro or nucleophile? C in CH3Br N in NH3 O in HO-arrow_forward10) What primary metabolites are the building blocks of this natural product Br Brarrow_forwardDraw the structure of the product.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY