
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Please help
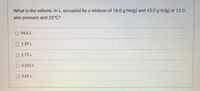
Transcribed Image Text:What is the volume, in L, occupied by a mixture of 16.0 g Ne(g) and 42.0 g Ar(g) at 15.0
atm pressure and 25°C?
O 94.6 L
O 1.29 L
O 1.71 L
O 0.252 L
3.01 L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- > 1arrow_forwardpart A 1. Concentration of Known NiCl2 Stock Solution ___500________ mM Tube Number Blank #1 #2 #3 #4 #5 2. Volume of Known NiCl2 Stock Solution Pipetted Into Tube 0.00 mL 1.50 mL 3.00 mL 4.50 mL 6.00 mL 7.50 mL 3. Volume of Distilled Water Pipetted Into Tube 11.00 mL 9.50 mL 8.00 mL 6.50 mL 5.00 mL 3.50 mL 4. Total Volume of Solution In Tube 11.00 mL 11.00 mL 11.00 mL 11.00 mL 11.00 mL 11.00 mL 5. Diluted Concentration of NiCl2 In Tube ----------- 68.2 mn 136mn 204mn 273mn 341mn 1. Equation for the Best-Fit Straight Line Y=3822x+4.7446 2. Absorbance at λmax for Diluted Unknown 0.87 3. Concentration of NiCl2 in Diluted Unknown 25.693 mM Determine the dilution factor for your diluted unknown U1 based on how you prepared the solutions in Part A. Record this on your report sheet in Part D #4.arrow_forwardhelpp??arrow_forward
- Can someone please help?arrow_forwardCan someone check to see if #9 through #12 are correct with the information providedarrow_forwardA chemist prepares a solution of silver perchlorate (AgCIO, by measuring out 1.07 kg of silver perchlorate into a 250. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's silver perchlorate solution. Round your answer to 3 significant digits. I mol L 10 Ar Explanation Check 2022 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center ACcessibility 80 F 8.37 AM Cloudy 令 4/02022 DELL F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 PrtScr Insert Delete PgUp PgDn Hom %23 V. Num Lock Backspace | 3 4 8 %24arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY