
University Physics Volume 1
18th Edition
ISBN: 9781938168277
Author: William Moebs, Samuel J. Ling, Jeff Sanny
Publisher: OpenStax - Rice University
expand_more
expand_more
format_list_bulleted
Question
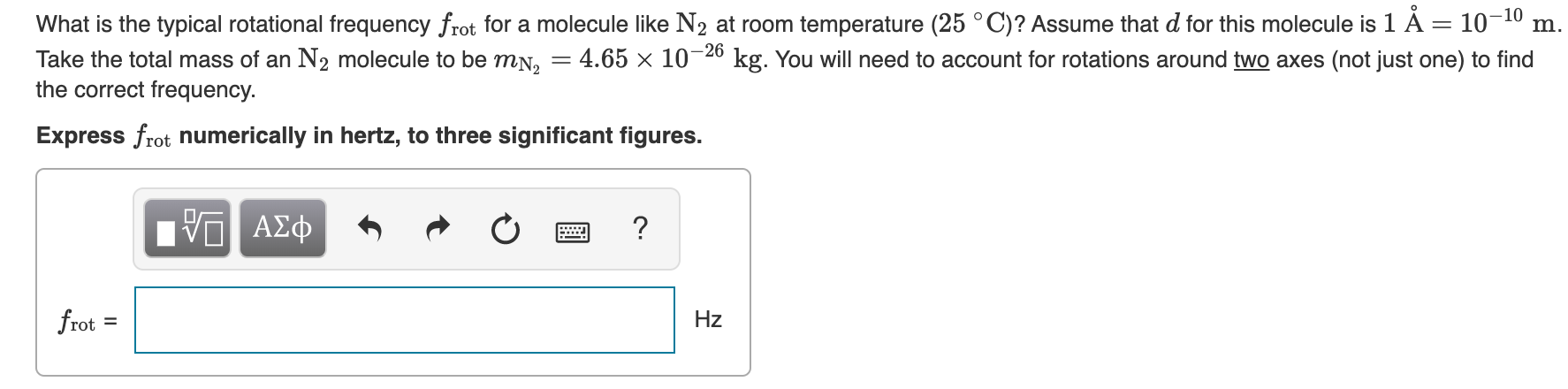
Transcribed Image Text:What is the typical rotational frequency frot for a molecule like N2 at room temperature (25 °C)? Assume that d for this molecule is 1 À = 10-10
Take the total mass of an N2 molecule to be mN,
m.
kg. You will need to account for rotations around two axes (not just one) to find
4.65 x 10-26
the correct frequency.
Express frot numerically in hertz, to three significant figures.
nν ΑΣφ
?
frot
Hz
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 6 images

Knowledge Booster
Similar questions
- Po = 25 μc/m³ is distributed uniformly through a certain region. If V = 50 at y = 2 and V= 90 at y = 5, &, = 2. a- Give the solution and calculate V, E.arrow_forwardPo = 25 uc/m³ is distributed uniformly through a certain region. If V=50 at y = 2 and V= 90 at y = 5, &, = 2. a- Give the solution and calculate V, E. b- Draw V, Ē, IE with respect to y-axis. c- Now if p₂ = 0, give the solution and calculate V, Ē.arrow_forwardThe density of platinum is 21.45 x 103 kg/m3. a. Calculate the volume (in m3/atom) occupied per platinum atom b. Estimate the atomic diameter (in m);(The estimate uses the approximation that it is a cubic volume) c. Using this estimation, calculate the thickness of a metal foil (in m) containing 2.0 x 101 atomic layers of platinum.arrow_forward
- This was wrong. Can you solve this again with these numbers? What is the root mean square velocity, vrms, for Hydrogen molecules (H2) at 20oC? Hint: How many amu does an H2 molecule contain. 1 amu = 1.67 x 10-27 kg Boltzman's Constant, k = 1.38 x 10-23 J/K Give your answer in m/s to 4 significant figures (NO DECIMALS)arrow_forwardWhich option is irarrow_forwardHi, could I get some help with this macro-connection physics problem involving the Ideal Gas Law? The set up is: What is the average volume in nm3 (cubic nanometers) taken up by molecules of an ideal gas at room temperature (taken as 300 K), and 1 atm of pressure or 101325 N/m2 to 4 digits of precision if kB = 1.38e-23 J/K and 1 nm = 10-9 m? Thank you.arrow_forward
- (a) A person’s blood pressure is measured to be 1202mmHg . What is its percent uncertainty? (b) Assuming the same percent uncertainty, what is the uncertainty in a blood pressure measurement of 80 mm He?arrow_forward(a) In the deep space between galaxies, me density of atoms is as low as 106atoms/m3, and me temperature is a frigid 2.7 K. What is me pressure? (b) What volume (in m3) is occupied by 1 mol of gas? (c) If this volume is a cube, what is the length of its sides in kilometers?arrow_forwardC L You are tasked with specifying the following structure. It is a cylinder attached to a sphere at one end. Unfortunately, you were not given all the dimensions. You know that the radius of the cylinder is one half (1/2) the radius of the sphere. What is the total volume of the structure as a function of the radius and length of the cylinder (you may neglect any excess volume at the join between sphere and cylinder). O a. V = πr²h+32r²/3 O b. V = r²(h+32r/3) O c. V = r²(th+32r/3) O d. V = r²(h+32/3)arrow_forward
- Hi, could I get some help with this micro-macro connection physics problem involving the rotational frequency of a molecule? The set up is: Treating the diatomic oxygen molecule O2 as a perfect dumbbell with length 0.12 nm between the molecules, what is the rotational frequency of the molecule in gigahertz (GHz) at a cold temperature of 100 kelvin (K) to 4 digits of precision if kB = 1.38e-23 J/K and the mass of O2 is 16 u, where the atomic mass unit u = 1.66e-27 kg? Thank you.arrow_forward1%A9 li. t LTET 4G+ Vol) O A X O U 9:-7 Second H.W.pdf 1. Draw and label the vector with these components. Then determine the magnitude and angle of the vector. a- Az = 3 ,A, = -2 b- B, = -2 ,B, = 2 2. What is the speed of horse in meter per second that runs a distance of 1.2 miles in 2.4 minutes? 3. A car moving along X- axis according on the function: X (t) = 2 t2 + 5t +7 on [1,2] Find: a- The average velocity. b- The acceleration at 3s. 4. Julie is walking around a track at a 2m/s for some exercise. She then decides to start jogging so she accelerates at a rate of 0.5m/s? for 3 seconds. How far did Julie travel from the time she started to accelerate to the end of the 3 seconds? 5. Ralph uses a 2000 to do this. force to push his car along a road for 1000 m. It takes him500 s a- Calculate the amount of work that Ralph did on the car. b- Calculate the amount of power that he generated. IIarrow_forwardFor this problem, we want to estimate the answer, so our assumptions may be a little unrealistic. Suppose we want to estimate how much air we need to send to a space station, assuming we cannot recycle air. Suppose four astronauts are in a spherical space station. If each of them typically breathes about 500 cm³of air with each breathe, and take 15 breathes per minute (average resting value): a. What is the volume of air you would need in the space station if these four astronauts stayed for a full year? b. If the density of air is 1.25 kg/m³ and it costs (thanks to SpaceX) a mere $100/kg to send objects to the space station, how much money would the astronaut air supply cost?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
