
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
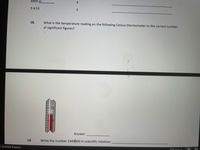
What is the temperature ready on the following Celsius thermometer to the correct number of significant figure
Transcribed Image Text:8900 m
4.
$ 4.53
What is the temperature reading on the following Celsius thermometer to the correct number
of significant figures?
18.
35
Answer:
19.
Write the number 1340000 in scientific notation:
O FOCIS
¬ (United States)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Is there easy way to figure out conversion factors? In simple terms can you explain conversion factor for metric system conversions from one unit to another?arrow_forwardUsing at least 2 conversion factors in sequence, concert the mass of a 254 mg aspirin tablet to Mg.arrow_forwardWhat is the maximum mass of pure copper that could be extracted from 7.5kg of chalcopyrite, a copper ore with the chemical formula CuFeS2? Be sure your answer has a unit symbol, if necessary, and is rounded to the correct number of significant digitarrow_forward
- Ammonia NH3 chemically reacts with oxygen gas O2 to produce nitric oxide NO and water H2O . What mass of oxygen gas is consumed by the reaction of 3.0g of ammonia? Be sure your answer has the correct number of significant digits.arrow_forwardState the number of significant digits in each of the following. a) 5.02 x 10^-1 mL b) 1.0 x 10^-2 sarrow_forwardRewrite the following statement using a number in scientific notation. The diameter of a certain atom is about 0.67 nanometersarrow_forward
- To the appropriate number of significant figures, what is the initial temperature of 3.liter of it absorbs 9900 Jul of energy. The final temperature of the water is 29.5 Celsius.arrow_forwardA brand new, unopened gallon container strawberry "-Flavored" milk , when weighed in its plastic container with the , weighs 9.575 pounds After someone drinks all the milk from the container , they weigh the empty container and the lid and determine that the combined weight of the empty container and lid is 0.175 pounds What is the density of the strawberry milk , expressed as grams per ml? Express your answer using the correct number of significant figuresarrow_forwardHow many low-dose 81 mg aspirin tablet can be made from 1.21 kg of aspirin? Do not answer in scientific notation. Enter only the numerical answer with the appropriate number of significant figures.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY