
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
What is the structure of C7H14O2 given the information. Also, what would happen if the structure were to change?
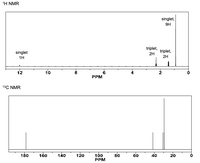
Transcribed Image Text:The image contains two nuclear magnetic resonance (NMR) spectra, specifically a ^1H NMR spectrum and a ^13C NMR spectrum.
### ^1H NMR Spectrum
- **X-axis (PPM):** Chemical shift in parts per million (PPM).
- **Y-axis (Signal Intensity):** Relative intensity of the signals.
- **Peaks:**
- **At ~12 PPM:** A singlet peak corresponding to 1 hydrogen (1H).
- **At ~4.6 PPM:** A triplet peak corresponding to 2 hydrogens (2H).
- **At ~1.2 PPM:** A triplet peak corresponding to 2 hydrogens (2H).
- **At ~0.9 PPM:** A singlet peak corresponding to 9 hydrogens (9H).
### ^13C NMR Spectrum
- **X-axis (PPM):** Chemical shift in parts per million (PPM).
- **Y-axis (Signal Intensity):** Relative intensity of the signals.
- **Peaks:**
- A single peak near 180 PPM.
- Multiple peaks between 40 and 20 PPM.
These spectra are used to identify chemical environments of hydrogen and carbon atoms within a molecule, allowing determination of the molecular structure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- explain to me what Isosteric replacements are when talking about strategies for lead molecule modification?arrow_forwardwrite the chemical formula for each and determine the class of compound of each of the structuresarrow_forwardHow many grams of glucose would need to be metabolized to produce this quantity of CO2? Assume that an exhaled breath of air consists of 74% N2, 15.5 %O2, 3.7 CO2, and 6.2% water vaporarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY