
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
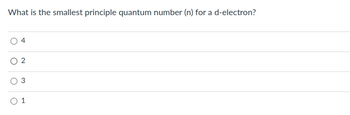
Transcribed Image Text:What is the smallest principle quantum number (n) for a d-electron?
4
o
♡
0 1
Expert Solution
arrow_forward
Step 1
The question is based on the concept of quantum numbers.
we need to identify lowest possible value of n for a d-electron
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 15arrow_forward62. What are quantum numbers? What information do we get from the quantum numbers n, I, and m/?arrow_forwardList the values of all four quantum numbers for each of the valence electrons of radium (Ra). State how these two sets of quantum numbers satisfy Pauli exclusion principle. List all the possible values of magnetic quantum number for the electrons in praseodymium? How many magnetic quantum number values are possible for the electrons in the energy level that is closest to the nucleus of praseodymium? List the values.arrow_forward
- Complete the table below by filling in the principal quantum number n and angular momentum quantum number / for each electron subshell listed. subshell 4f 4d 7p 6s principal quantum number n 1 7 angular momentum quantum number / 1 0 0 X Śarrow_forwardAn electron initially in a 4p state decays to a lower energy state. Which energy state is forbidden? 2p 1s 2s 3darrow_forwardThe Auf Bau Principle, Hund's rule, and the Pauli exclusion principle are used to determine the lowest energy electron configuration of an atom, called the ground state; any other possible configuration is higher in energy, an excited state. Which of the following valence shell electron configurations are excited states? 5s 5px 5py 5pz 4dz2 4dx2-y2 4dxy 4dyz 4dxz (1) up, down up up up (2) up, down ¯ up up (3) up, down up up up (4) ¯up, down up up up 1, 2, and 3 (b) 1 and 4 (c) 2 only (d) 4 only (e) 3 only answer is B. i need explanation.arrow_forward
- Hi! Wouldn't there only be five answers (and not 10) for the quantum numbers since the electrons in the d-orbitals are unpaired?arrow_forwardThe n quantum number of an atomic orbital is 5. What are the possible values of l? What are the possible values of me if the & quantum number is 5?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY