
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
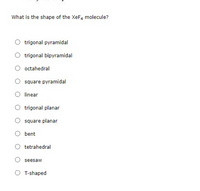
Transcribed Image Text:What is the shape of the XeF4 molecule?
trigonal pyramidal
trigonal bipyramidal
octahedral
square pyramidal
linear
trigonal planar
square planar
bent
tetrahedral
seesaw
O T-shaped
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Given that the F-CI-F bond angles in CIF3 are 180° and 90°, predict the geometry of the molecule. seesaw trigonal pyramidal trigonal planar tetrahedral none of the abovearrow_forwardWhat is the molecular geometry of SOCl2?arrow_forwardWhat orbital would the lone pair on the central oxygen of ozone (O3)be located?arrow_forward
- Why do atoms promote electrons and hybridize their orbitals before bonding to other atoms?Explain using CH4 as an examplearrow_forwardFor the following descriptions of molecules, draw the Lewis structure (showing all atoms, lone pairs, formal charges) of the molecule and show all bond angles (assuming ideal VSEPR angles). An organic compound with the molecular formula H3C2NO. One C is sp hybridized, while the second C is sp3 hybridized, and both N and O are sp? hybridized.arrow_forward6. Which of the following structure(s) is/are chiral? Br Br Br 1 Br 2 Br Br 3 Br Br 4arrow_forward
- Consider the structure of C103. Part 1 of 3 What is the molecular shape? Select the single best answer. square planar bent O trigonal bipyramidal O trigonal planar seesaw linear trigonal pyramidal square pyramidal O T-shaped octahedral tetrahedral Part 2 of 3 What is the ideal bond angle? Part 3 of 3 O X ideal What, if any, is the direction of deviation from the ideal bond angle? Select the single best answer. X greater than ideal less than ideal Xarrow_forwardWhat is the hybridization of the carbon atom indicated with a star in the given structure?arrow_forwardThe correct name for the covalen molecule, N5C17 is:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY