
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
what is the purpose of the water trap that is connected in the condenser in the set up shown below
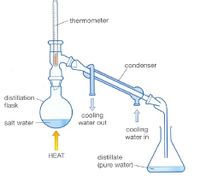
Transcribed Image Text:-thermometer
çondenser
distillation
flask
cooling
salt water
water out
cooling
water in
HEAT
distillate
(pure water) -
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q: sintering means: a.Shock cooling of molten glass to make frits. b.The molecular bonding of ceramic particles below their melting point. c.Melting of non-refractory clay constituents. d.Condensation of constituent particles to form greenware.arrow_forwardSee image below. Table provided is for this question. I want to double check to see if my answer is correct!arrow_forwardWhat is the volume of a ceramic evaporating dish closest to? Question 9 options: 25 mL 5 mL 150 mLarrow_forward
- please ....arrow_forwardLiquid A is known to have a lower surface tension and lower viscosity than Liquid B. Use these facts to predict the result of each experiment in the table below, if you can. experiment Small amounts of Liquid A and Liquid B are sprayed into the air, where they form perfect spheres with a volume of 20.0 µL. The diameters of these drops are measured with a high-speed camera, and their surface areas S and S calculated. A 20.0 mL of Liquid A are poured into a beaker, and 20.0 mL of Liquid B are poured into an identical beaker. Stirrers in each beaker are connected to motors, and the forces F and F needed to stir each liquid A В at a constant rate are measured. predicted outcome OS will be greater than Sp will be less than SB OS will be equal to SB It's impossible to predict whether SA or SB will be greater without more information. FA will be greater than FB А OFA will be less than FB F will be equal to F B It's impossible to predict whether For Fg will B be greater without more…arrow_forwardplease help mearrow_forward
- V5arrow_forwardThis next picture shows 6 more substances to be tested. These will be burned in solid form. Note that there are 3 elemental powders, small pieces of charcoal, a wooden splint, and a piece of steel wool. Selenium powder Sulfur Copper powder Charcoalarrow_forwardIt is also important to place a boiling stone in the round bottom flask (or use a stir vane). Why do you think this is necessary?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY