
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
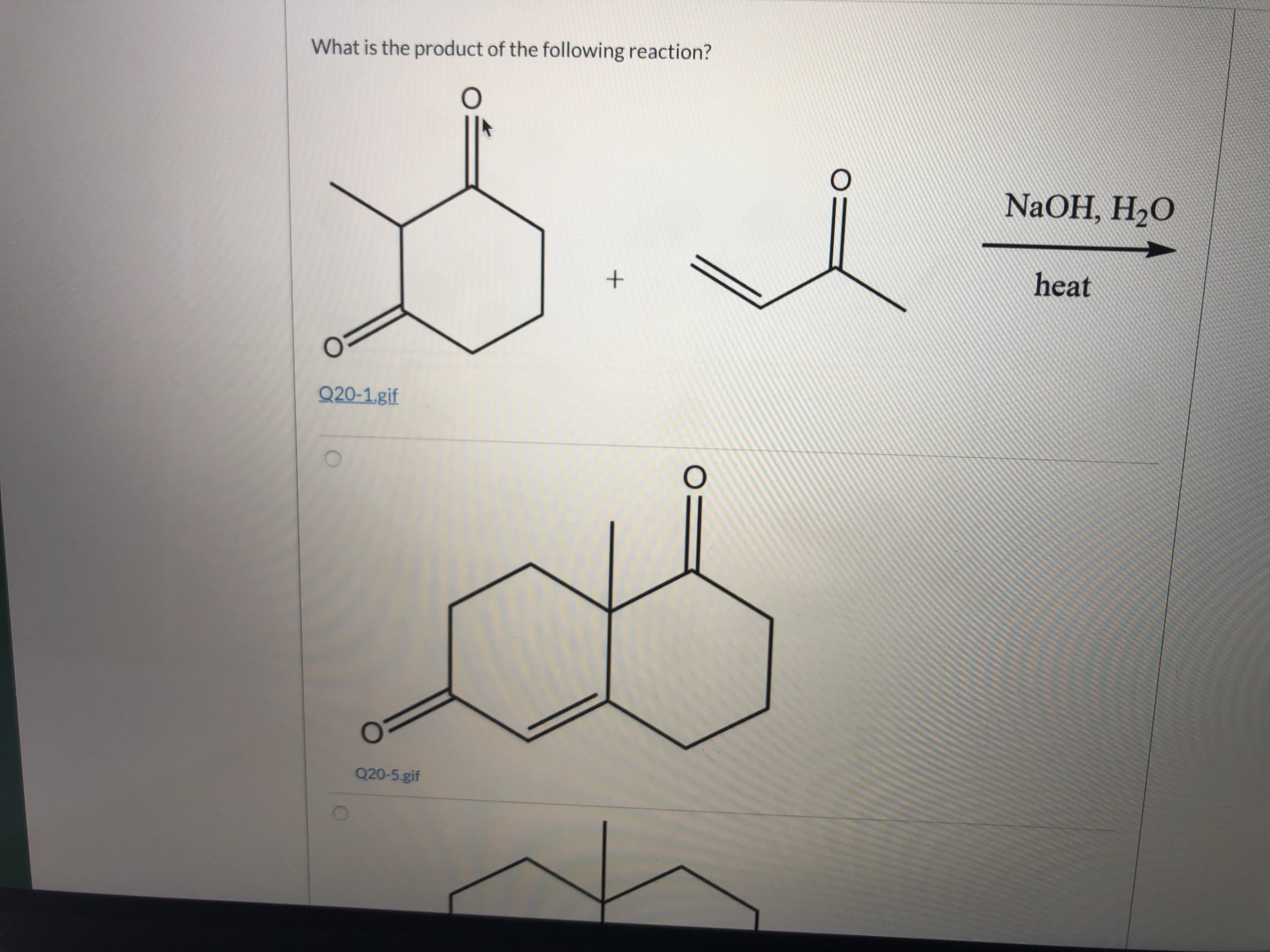
Transcribed Image Text:What is the product of the following reaction?
NaOH, H2O
heat
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the correct oxidation product ?arrow_forwardShow structures (in blank boxes) for the reactants/products/reagents in the following reactions.arrow_forwardWhich of the following represents the balanced equation for the combustion of Pentane? 2 CH3(CH2)2CH3 + 13 O2 → 8 CO2 + 10 H₂O CH3(CH2)3CH3 + 8 O2 → 5 CO2 + 6 H₂O CH3(CH2)3CH3 + 7 O2 → 5 CO2 + 6H₂O + 16 O2 → 10 CO2 + 12 H2O + 26 O2 →16 CO2 + 20 H₂O 2 CH2(CH2)3CH3 4 CH3(CH₂)3CH3arrow_forward
- For the following reactions of one mole ofreactant, predict the product, including its structuralformula and name, and state the chemical amount (inmoles) of hydrogen required for complete reaction.CH2CHCHCH2 + ___ H2(g) →arrow_forwardWhat is the major product of the following reaction? Na Cr,O,, H,SO,H,0 OHarrow_forwardPredict the products of the following reaction. If no reaction will occur, use the NO REACTION button. Be sure your chemical equation is balanced! →+AlsH2Olarrow_forward
- col H₂C H Consider the organic structures shown below. Which one corresponds to an aromatic ketone? CH3 CH3 & C. B. CH,CHCH T F. CH, CHCH₂ NH₂ CH3 H CH, CH3 Question 17 of 20 H A) Compound A B) Compound C C) Compound D D) Compound E E) Compound G Ⓒa-parrow_forwardClassify the following reaction: H₂CO₃(aq) → H₂O(l) + CO₂(g) A) synthesis B) decomposition C) combustion D) single displacement (replacement) E) double displacement (replacement)arrow_forwardWhat are the products of the following reaction?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY