
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
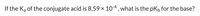
Transcribed Image Text:If the Ka of the conjugate acid is 8.59 x 10-4, what is the pKb for the base?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An aqueous solution of HNO3 has pH 4. What is the pOH of the solution? Answer 1Choose...neutral1x10^-31x10^-4base10Acid1x10^-141x10^-10 The solution is Answer 2Choose...neutral1x10^-31x10^-4base10Acid1x10^-141x10^-10 What is the Hydroxide ion concentration of the solution Answer 3Choose...neutral1x10^-31x10^-4base10Acid1x10^-141x10^-10 What is the Hydrogen ion concentration of the solution Answer 4Choose...neutral1x10^-31x10^-4base10Acid1x10^-141x10^-10arrow_forwardA good buffer system has the Ka of the weak acid as close to as possible to the desired pH of the buffer. c) True Falsearrow_forwardydrangea plants produce flowers that can be either blue or pink in color. the flowers will be blue if they are grown in acidic soil (having a ph of 5.5 or less) or pink if they are grown in soil with a neutral to basic ph of 6.5 and up. what can be concluded from this information?arrow_forward
- The theoretical pK, of a weak acid is 3.54. Calculate the pK, experimental error of a solution composed of 10 mL of 0.23 M HA, 15 mL of 0.17 M of NaA and 20 mL of water. The measured - of C pH of this solution is 3.17. (Report only the numerical answer of the percent without the % symbol)arrow_forwardA 1.23M solution of an unknown weak base has a pH of 11.5. What is its Kb?arrow_forwarda .1000 M solution of a weak acid, HA, is 3.0% dissociated. Determine the value of Ka for the weak acidarrow_forward
- Will addition of HCl to a solution make it more acidic or more basic? Will the pH increase or decrease?arrow_forwardIf you titrate 41.32ml of 6.4M NaOH with 21.34ml of 2.125M HCl, the resulting solution would still be basic. HOw many moles of OH- are left in the solution and what is the new OH- concentration?arrow_forward3. What is the pH of a solution that is 0.75 M in sodium acetate and 0.50 M in acetic acid? (K, for acetic acid is 1.8x10-5.)arrow_forward
- Equal molar amounts of benzoic acid and sodium acetate are mixed together in water, is the resulting solution acid,basic, or neutral? Here are some Ka valuesarrow_forwardWhy do successive Kₐ’s decrease for all polyprotic acidsarrow_forwardAn aqueous 0.6 M solution of a weak base has pOH of 4.5, what is the pKb of the weak base?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY