
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
What is the peroxide effect? What does it mean when there is a reversal of orientation? Detailed answers are appreciated. Thanks in advance!
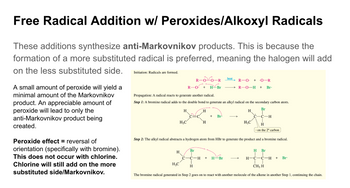
Transcribed Image Text:Free Radical Addition w/ Peroxides/Alkoxyl Radicals
These additions synthesize anti-Markovnikov products. This is because the
formation of a more substituted radical is preferred, meaning the halogen will add
on the less substituted side.
A small amount of peroxide will yield a
minimal amount of the Markovnikov
product. An appreciable amount of
peroxide will lead to only the
anti-Markovnikov product being
created.
Peroxide effect = reversal of
orientation (specifically with bromine).
This does not occur with chlorine.
Chlorine will still add on the more
substituted side/Markovnikov.
Initiation: Radicals are formed.
R
R-O: +
Propagation: A radical reacts to generate another radical.
Step 1: A bromine radical adds to the double bond to generate an alkyl radical on the secondary carbon atom.
Br
H
H₂C
Br
H
A
C-C
H
H
H3C
H
-R
-Br
+ Br
-H +
heat
R-O + ·O-R
R-0-H + Br.
H-Br
H
Step 2: The alkyl radical abstracts a hydrogen atom from HBr to generate the product and a bromine radical.
H3C
C-H
H Br
T
H-C-C-H + Br.
CH3 H
The bromine radical generated in Step 2 goes on to react with another molecule of the alkene in another Step 1, continuing the chain.
H
on the 2° carbon
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please provide a justification for the following statement: lithium chloride dissolves in polarorganic solvents. What would you expect for other group I metal halides?arrow_forward4. Briefly distinguish between the circumstances for the use of nRln(V2/V1) and CvIn(T2/T1) in calculating delta S.arrow_forwardHow and what is the mechanism by which crystal structure of the mineral is altered by calcination , which in turn can impact its surface acidity. What happens when a crystal structure changes and why does it affect surface acidity?arrow_forward
- Provide mechanisms for the reactions below, Include relevant resonance structures to describe charge stabllization.arrow_forwardNonearrow_forwardIn the formation of phenyl-isothicyanite, orbitals on C, S, and N overlap to form bonds involving these elements. N - sp2; C – sp3; S – sp3 ON-sp2; C – sp3; S - sp3 ON- sp; C - sp; S – sp3 ON- sp2; C – sp2; S – sp2arrow_forward
- “Mango-globin” is a hemoglobin variant in which the iron atoms in each subunit have been substituted with manganese atoms. Mango-globin binds to oxygen at very high partial pressures, but does not exhibit any cooperativity. Propose an explanation for this observation. (Hint: the ionic radius of Fe2+ is 77 pm; the ionic radius of Mn2+ is 82 pm.) The free energy change for a chemical reaction is defined as follows: ∆?!= ∆? ̊!+ RT ln [products] / [reactants] When a reaction is at equilibrium, ∆G’ = 0, and this expression simplifies to: ∆? ̊!= −RT ln [products] / [reactants] At equilibrium, the ratio of product to reactant concentrations is nothing more than the equilibrium constant, Keq, for the reaction. Under these conditions: ∆G ̊’ = -RT ln Keqarrow_forwardDraw the reaction coordinate diagram for a reaction that is thermodynamically favoured, but kinetically disfavoured. Give an example discussed in class of such a reaction for an allotrope of a Group 14 element (not a molecule). Why is the reaction kinetically unfavourable? What thermodynamic factors FAVOUR the reaction, in Gibbs-Helmholtz terms, and why?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY