
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
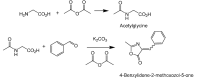
What is the percent yield of 4-benzylidene-2-methoxozol-5-one relative to glycine
- Show all calculations (including determining limiting reagent and balanced equations)
- Maximum percent yield for each step is 100%
Information:
- Start with 0.62g of glycine
- 1.5mL of acetic anhydride
- 3mL of water
- Acetylgycine product measured: 0.61g
- Used all acetylglycine product in next step
- Measured 0.43g of potassium carbonate
- Added 3mL of acetic anhydride
- Added 0.60mL of benzaldehyde
- Crude product measurement: 1.04g
- Pure product isolated: 0.18g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 10 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- According to the proposed schemes, write the equations of reactions. Be sure to write down the formulas of substances Methanic acid formation from lactic acid.arrow_forward3arrow_forwardWhat is the atom economy % of 0.2529g of 3,4-dimethoxybenzaldehyde and 0.2067g of 1-Indanone. What is the atom economy % of 0.0512g of 2-4 hexadiene and 0.0515g of maleic Anhydride.arrow_forward
- What is the experimental yield of CRUDE PRODUCT AND FINAL PRODUCT (grams and percentage) Experiment: Oxidation of -Chlorotoluene to o-Chlorobenzoic acid materials used dissolved 3.35 g of KMnO4 1.20 mL of 2-chlorotoluene was added to the mixture 3.0 mL of concentrated hydrochloric acid (pH~2) 10 mL of toluene -The crystals were isolated by vacuum filtration- Product Characterization 1.15 g of the crude product 0.93 g of the final product M.p. (final): 139-141 celsiusarrow_forward6. Make a table with the physical properties (molecular mass, b.p., m.p., density, solu- bility, flammability [for solvents only], and toxicity/hazards) of caffeine, methylene chloride, calcium carbonate, acetone, and petroleum ether.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY