
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What is the percent Chloride in in a sample of 3.7 g of the sample produced 2.7 g of CdCl2 when treated with excess Cd+2?
Cd+2 + 2Cl -> CdCl2
Expert Solution
arrow_forward
Step 1
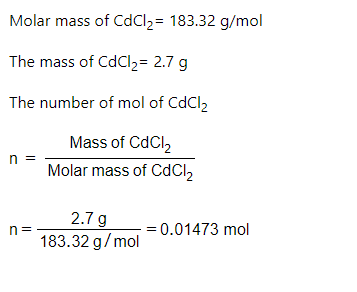
arrow_forward
Step 2
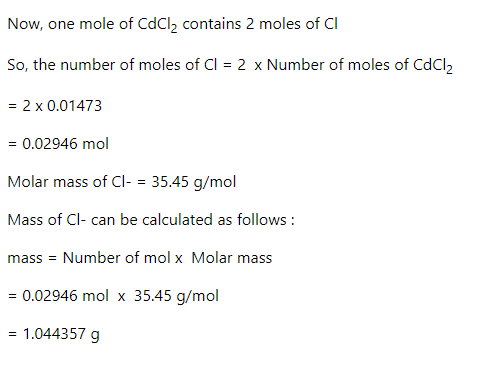
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If 0.90 mol of H2 reacts with 0.25 mol of CO, how many moles of CH4 will be formed? 3H2 + CO ---> CH4 + H2O Question 4 options:arrow_forwardA cvg.cengagenow.com/ilrn/takeAssignment/takeCXPCompliantActivity.do?locator=Dassignment-take CHAPTER 4 - STOICHIOMETRY: QUANTITATIVE INFORMATION ABOUT CHEMIC Page 1 of 9 Next If 5.68 g of Na, CO3 is dissolved in enough water to make 250. mL of solution, what is the molar concentration of the sodium carbonate? Molar concentration of NazCO3 = M What are the molar concentrations of the Na and CO ions? Molar concentration of Na = Molar concentration of CO,arrow_forward10 g H2O reacts with 4.5 g Na to produce NaOH and H2arrow_forward
- Using the balanced equation below to determine the molar ratio between the chemical pair: O2 and H2O C3H8+ 5O2 --> 4H2O + 3CO2arrow_forwardIt is also possible to calculate the % CO2 by dividing the molar mass of the product CO2 by the molar mass of the particular carbonate used. Show this version of the calculation for Na2CO3, and then for KHCO3arrow_forwardWhat is the percent Chloride in in a sample of 3.7 g of the sample produced 2.7 g of CdCl2 when treated with excess Cd+2? Cd+2 + 2Cl -> CdCl2arrow_forward
- Data Table 2: Alum Data Object Mass (g) Aluminum 2.38 g Cup (Empty) Aluminum Cup + 2.0 grams of Alum Aluminum Cup + Alum After 1st Heating Aluminum Cup + Alum After 2nd Heating Mass of Released 4.38 g 3.48 g 3.48 g 0.900 garrow_forwardWhat is the percent Chloride in in a sample of 3.7 g of the sample produced 2.7 g of CdCl2 when treated with excess Cd+2? Cd+2 + 2Cl -> CdCl2arrow_forwardWhat is the percent Chloride in in a sample of 3.7 g of the sample produced 2.7 g of CdCl2 when treated with excess Cd+2? Cd+2 + 2Cl -> CdCl2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY