
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
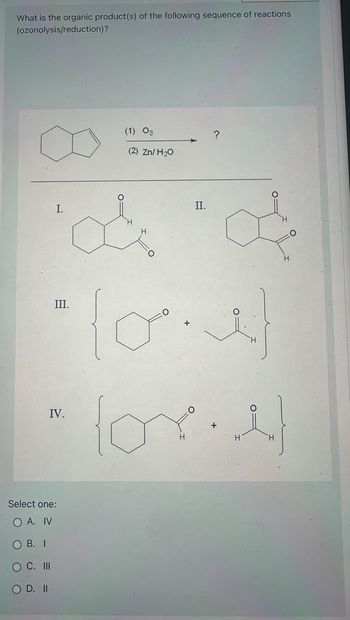
Transcribed Image Text:What is the organic product(s) of the following sequence of reactions
(ozonolysis/reduction)?
I.
III.
IV.
C. III
O D. II
Select one:
O A. IV
OB. I
(1) 03
(2) Zn/H₂O
II.
?
i
O
(ore)
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please don't provide handwritten solutionarrow_forwardv2/assessment-player/index.html?launchld=e7f9ff7e-311b-440d-8832-4aeb728ebe50#/question/28 3 WileyPLUS HW Question 29 of 30 View Policies Current Attempt in Progress What is the likely product of the reaction shown? 1. CH3ONA 2. Hао CH3 OH CH3 OCH3 CH3 nCH3 "OCH3 "OCH3 OCH3 IV "OH |3| II O II O IV O none of these eTextbook and Media W SS DELLarrow_forward2. Draw the product, if any, for each of the following reactions. If no change occurs, write "No reaction". a. Pt + H₂- -> b. [0] C. Pt + H2- d. [0]arrow_forward
- Question 4 Please predict the products for each of the following reactions: Hint: Treat D (deuterium atom) similar to H (hydrogen atom). 1. 03 2. H₂O NaNH, 10 1. n-BuLi 2. Mel H₂SO4, H₂O HgSO4 H2 Pd or Pt (catalyst) B Lindlar's Catalyst D₂ (deuterium) n-BuLi 1. NaNH, 2. Eter 4 Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)₂BH C D to to to to ○ A ABCDarrow_forward# 3 E D C Predict the product of the given reduction reaction. Include all hydrogen atoms. o H₂C H₂C. 28 $ 4 R F - V H₂ CH₂ + H₂- CH₂ % 5 T G B I 6 Pt H Modify the molecule so that it represents the product of the reaction. You may need to add or remove bonds or atoms. Draw Templates More N Select MacBook Pro 9 |||||||Co H 27 U J . 8 M TEZA I O K ! 9 H < O L command 04 0 CH, CH₂ P A : ; I option - Q2Q { [ ? / Aa + - Erase " } 1 delete return shiftarrow_forwardBelow is the answer . Could I get an explanation please.arrow_forward
- r. Pleasse don't provide handwriting solution.arrow_forwardDraw all the products formed and balance each reaction. Assume H; O*C work-up. .arrow_forwardWhat is/are the product/s of the following metathesis reaction? You can use either of the catalysts below. QA 2 H CF3 H C=C CF3 + CH3 CF3 2013 Pearson Education, inc CH3 =N -0-Mo-C O CH3 CF3 catalyst (a) Schrock H Cl. CI P Ru P. (b) Grubbs C Harrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY