
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
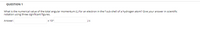
Transcribed Image Text:QUESTION 1
What is the numerical value of the total angular momentum (L) for an electron in the f sub-shell of a hydrogen atom? Give your answer in scientific
notation using three significant figures.
Answer:
x 10
Js
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A photo with the nervy of 9.08 x 10^-15 Joules has what frequency, in Hertz (Hz)?arrow_forwardFour research teams measured the rotation period of a newly detected neutron star, and what each team wrote in its team notebook is shown in the table below. Suppose a later and more reliable measurement gives 0.42 s for the period of the same star. Decide which of the earlier measurements was the most accurate, and which was the most precise. what was written most accurate most precise team in the notebook ? measurement measurement A "0.22s" B "0.4s + 0.1s" "0.52s + 10.%" D "between 0.30s and 0.34s"arrow_forwardWhat is the frequency observed with a wavelengh of (4.0x10^-7) m? Answer with 2 significant figures and it must be in scientific notation. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: x10arrow_forward
- Alejandra just finished making her hydrogen calibration curve. Her data is best described by the following equation: ?=−127.4ln(?)+715.23y=−127.4ln(x)+715.23 If she observed a line in the helium spectrum that had a scale reading of 5.91 what wavelength, in nm, would this correspond to? in sig figsarrow_forwardCalculate the energy in kJ/mol of a mol of photons in the microwave region of the electromagnetic spectrum that has a frequency of 1.65 × 1011 Hz. Express answer in scientific notation.arrow_forwardPlease answer question 5 Part Aarrow_forward
- 1) Listen Find the wavelength (in nm) of a baseball of mass (6.40x10^-1) kg moving at (2.600x10^3) m/s. Answer to 3 significant figures and include the unit. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: х10 Answer unitsarrow_forwardA source of electromagnetic radiation emits light with a wavelength of exactly 225. nm. The energy of one photon of this light is ________ Joules. DO NOT WRITE THE UNITS; ONLY THE VALUE Please use the following format for scientific notation: a*10^n where a is the coefficient and n is the exponent.arrow_forwardif n=2 what are all of the allowed values of l and ml?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY