
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Zain JO lI.
Done
Edit
Imssb1.mutah.edu.jo
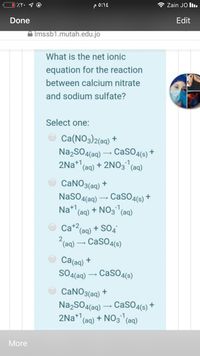
What is the net ionic
equation for the reaction
between calcium nitrate
and sodium sulfate?
Select one:
Ca(NO3)2(aq) +
Na,SO4(aq) – CaSO4(s) +
2Na* (ag) + 2NO3 (aq)
-1,
CaNO3(aq) +
NaSO4(aq) – CaSO4(6s) +
Na* (ag) + NO3(
(aq)
Ca+2(ag) + SO4
2(aq) – CaSO4(s)
Ca(aq) *
SO4(aq) – CasO4(s)
CANO3(aq)
Na,SO4(aq) – CaSO4(s) +
2Na+1
'(aq) + NO3 (aq)
More
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Physiological saline concentration—that is, the sodium chloride concentration in our bodies—is approximately 0.16 M . A saline solution fur contact lenses is prepared to match the physiological concentration. If you purchase 25 mL of contact lens saline solution, how many grams of sodium chloride have you bought?arrow_forwardThe formula for tartaric acid is preferably written as H2C4H4O6 rather than as C4H6O6. Explain why.arrow_forwardWhat does it mean to say that mercury (II) halides are weak electrolytes?arrow_forward
- 87. What volume of 0.151 N NaOH is required to neutralize 24.2 mL of 0.125 N H2SO4? What volume of 0.151 N NaOH is required to neutralize 24.2 n1L of 0.125 M H2SO4?arrow_forwardMagnesium metal (a component of alloys used in aircraft and a reducing agent used in the production of uranium, titanium, and other active metals) is isolated from sea water by the following sequence of reactions: Mg2+(aq)+Ca(OH)2(aq)Mg(OH)2(s)+Ca2+(aq)Mg(OH)2(s)+2HCl(aq)MgCl2(s)+2H2O(l)MgCl2(l)electrolysisMg(s)+Cl2+Cl2(g) Sea water has a density of 1.026 g/cm3 and contains 1272 parts per million of magnesium a5 Mg2+(aq) by mass. What mass, in kilograms, of Ca(OH)2; is required to precipitate 99.9% of the magnesium in 1.00103 L of sea water?arrow_forwardIf enough Li2SO4 dissolves in water to make a 0.33 M solution, explain why the molar concentration of Li+ is different from the molar concentration of Li2SO4(aq).arrow_forward
- The present average concentration (mass percent) of magnesium ions in seawater is 0.13%. A chemistry textbook estimates that if 1.00 × 108 tons Mg were taken out of the sea each year, it would take one million years for the Mg concentration to drop to 0.12%. Do sufficient calculations to either verify or refute this statement. Assume that Earth is a sphere with a diameter of 8000 mi, 67% of which is covered by oceans to a depth of 1 mi, and that no Mg is washed back into the oceans at any time.arrow_forwardWrite balanced chemical equations for the following reactions: (a) zinc metal heated in a stream of oxygen gas (b) zinc carbonate heated until loss of mass stops (c) zinc carbonate added to a solution 0f acetic acid, CH3CO2H (d) zinc added to a solution of hydro-bromic acidarrow_forwardWhat volume of 0.200 M NaOH is necessary to neutralize the solution produced by dissolving 2.00 g of PCl3 is an excess of water? Note that when H3PO3 i5 titrated under these conditions, only one proton of the acid molecule reacts.arrow_forward
- What is the total ionic reaction and net ionic reaction of Na2CO3 (aq)+ 2HCl(aq) -> 2NaCl (aq)+ CO2(g) + H2O(l)?arrow_forwardJust double checking to make sure my answer is correct? Thanksarrow_forwardPredict the products of the reaction (or look at the previous question if it is the same reaction). NaBr(aq) + F2(g) ----> i) Write the letter of the equation that represents the balanced total ionic equation for this reaction. A. Na+(aq) + Br-(aq) + F2(g) ---> Na+(aq) + FBr-(aq) + F(g)B. 2 Na+(aq) + 2 Br-(aq) + 2 F2(g) ---> 2 Na+(aq) + 2 F-(aq) + 2 FBr(l)C. 2 Na+(aq) + 2 Br-(aq) + F2(g) ---> 2 Na+(aq) + 2 F-(aq) + Br2(l)D. Na+(aq) + Br-(aq) + F2(g) ---> Na(s) + BrF2(l)ii) Write the letter of the equation for the net ionic equation of this reaction. A. 2 Br-(aq) + F2(g) ---> 2 F-(aq) + Br2(l)B. Br-(aq) + F2(g) ---> FBr-(aq) + F(l)C. Na+(aq) + Br-(aq) + F2(g) ---> Na(s) + BrF2(l)D. 2 Na+(aq) + F2(g) ---> 2 Na+(aq) + 2 F-(aq)iii) Which of the following describes the chemistry of this reaction? (choose the correct letter from A - D below.) A. Precipitation B. Redox C. Acid-Base Neutralization D. Neitherarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning