
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What is the net ionic equation and what type of reaction is this?
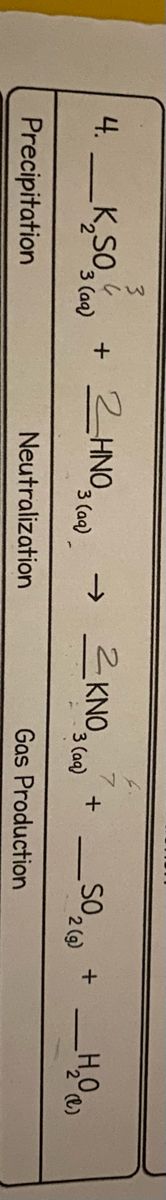
Transcribed Image Text:3
KNO3 (aa)
4. _K,S0, + 2INO, c.
+ 2HNO,
3 (aq)
_SO,
3 (aq)
2 (g)
Neutralization
Gas Production
Precipitation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write a net ionic equation for the reaction that occurs when aqueous solutions of ammonia and hydrobromic acid are combinedarrow_forwardwrite a net ionic equation for the reaction that occurs when barium carbonate (s) and excess hydrobromic acid (aq) are combinedarrow_forwardWrite the molecular equation for the reaction that occurs, if any, when solutions of the following substances are mixed: sodium bromide and lead nitratearrow_forward
- Does a reaction occur when aqueous solutions of manganese(II) chloride and silver(I) sulfate are combined? If a reaction does occur, write the net ionic equation.arrow_forwardEnter your answer in the box provided. A 2250 mL sample of 2.50 M HCl solution is treated with 4.14 g of magnesium. Calculate the concentration of the acid solution after all the metal has reacted. Assume that the volume remains unchanged. M HCI encesarrow_forwardA standard solution of 0.2436 M NaOH was used to determine the concentration of a hydrochloric acid solution. If 46.33 mL of NaOH is needed to neutralize 10.00 mL of the acid, what is the molar concentration of the acid in the original solution?arrow_forward
- 8. A chemist wants to make 100 of a 0.15 M AlCl 3 (aq) solution. How many grams of aluminum chloride should the chemist use?arrow_forwardWrite the net ionic equation for any precipitation reaction that may be predicted when aqueous solutions of sodium carbonate and chromium(II) nitrate are combined.arrow_forwardSome chemical reactants are listed in the table below. Complete the table by filling in the oxidation state of the highlighted atom. oxidation state of species highlighted atom Cro (aq) ОН (ад) NH(aq) co, (aq)arrow_forward
- V A chemistry student weighs out 0.135 g of phosphoric acid (H₂PO4), a triprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1200M NaOH solution. O CHEMICAL REACTIONS Determining the volume of base needed to titrate a given mass... Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Be sure your answer has the correct number of significant digits. icrosoft O W Welcome, Simrat - Microsoft tab os lock 0 Microsoft 6.52.210... esc mL control Explanation 1 Q A 2 option Z Check @ 2 W S #3 X command x10 X E D NOV 14 $ 4 C S R 8 F 07 dº % 5 T V tv MacBook Pro < 6 G Y & 7 H Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility U * 00 8 B N J "A ( 9 M K O W O L H P command 33 + 11 [ I optarrow_forwardDetermine the molecular equation for the reaction of zinc carbonate with hydrochloric acid. + Determine the net ionic equation for this reaction.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY