
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
38
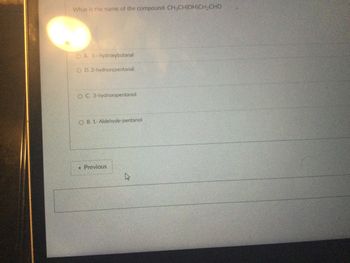
Transcribed Image Text:What is the name of the compound: CH₂CH(OH)CH₂CHO
OA. 3-hydroxybutanal
O D. 2-hydroxypentanal
O C. 3-hydroxypentanol
OB. 1- Aldehyde-pentanol
< Previous
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Decide whether each of the following is water-soluble. If soluble, tell what ions are produced. (Use one answer blank per ion. If not soluble, enter "none" in both answer blanks.) These are the solubility rules for the ionic compounds in water at room temperature: Rule Applies to Statement Li+, Na+, K+, Rb+, Cst, Group 1 and ammonium compounds are NH₂+ soluble. C₂H3O2, NO3-, NO₂- 1 2 3 4 5 6 7 8 9 10 Cl, Br, I F C1O3-, C104 SO4²- CO3²- 2- 3 PO4³ 8². OH Acetates, nitrates, and nitrites are soluble. Most chlorides, bromides, and iodides are soluble. Most fluorides are soluble. Most chlorates and perchlorites are soluble. Most sulfates are soluble. Most carbonates are insoluble. Most phosphates are insoluble. Most sulfides are insoluble. Most hydroxides are insoluble. Exceptions No common exceptions Moderately soluble: AgCH3COO and AgNO3 Insoluble: AgCl, Hg₂ Cl₂, PbCl₂, AgBr, Hg₂ Br₂, PbBr2, AgI, HgI₂, Pbl₂ Insoluble: MgF₂, CaF2, SrF2, BaF2, PbF2 No common exceptions Insoluble: BaSO4,…arrow_forwardhow do you calculate the mass of evaporating dish after removing iron fillings? And the mass of Fe?arrow_forward1. How many grams of Cu+ are present in 2.20 grams of copper(I) chromate? grams Cu+.2. How many grams of copper(I) chromate contain 3.24 grams of Cu+? grams copper(I) chromate.arrow_forward
- Please please answer both questions fastarrow_forwardBalance and complete: HNO3(aq)+K2CO3(aq) Show your work!arrow_forwardA student dissolves 106 g of compound Q in one liter of hot water. All of compound Q dissolves. The student then cools the water to room temperature. At room temperature, 52 g of compound Q dissolves in one liter of water. How many grams of compound Q will recrystallize in the room temperature water? Include the unit in your answer.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY