
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please show me every step to solve this problem!
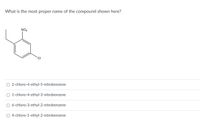
Transcribed Image Text:What is the most proper name of the compound shown here?
NO2
CI
O 2-chloro-4-ethyl-5-nitrobenzene
O 1-chloro-4-ethyl-3-nitrobenzene
O 6-chloro-3-ethyl-2-nitrobenzene
O 4-chloro-1-ethyl-2-nitrobenzene
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- BASIC PURE SUBSTANCE MIXTURE UNIT DESCRIPTION (color; physical state; transparent/translucent/opaque; shiny/dull malleable/ductile, shape and size of particles for solids; odor for organic series only) SAMPLE OF Соmpound (for Pure substances Element MATTER only) Homo- Hetero- atom, molecule, or formula unit Metal Nonmetal Ionic Covalent Series 8. ORGANIC CHEMICALS C,H14(1) Нехane HC,H3O2(aq) C4H3O2(1) ethyl acetate C;H60(1) Acetone CH,O(1) ethyl alcohol (ethanol) C10H3O(s) 1-naphthol C;H,O(1) Benzaldehyde C3H3O2(1) methyl benzoate C,H§O(I) trans-cinnamaldehydearrow_forward____________ makes up the majority of oil use, with gasoline and diesel fuel being the most common fuels for this genre. __________ makes up the majority of natural gas usage. Electricity makes up the majority of coal burning. Fill in the Blank!arrow_forward1. Did the slime pick up water soluble or water insoluble inks in? From these results, what can you conclude about the polarity of slime molecules? Explain. 2. Explain how you determined your hypothesis about whether or not the slime would pick up water soluble inks. What scientific information did you incorporate to formulate the hypothesis? Was your hypothesis correct?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY