
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
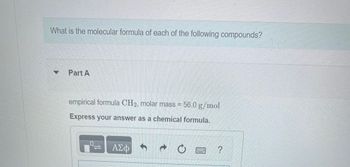
Transcribed Image Text:What is the molecular formula of each of the following compounds?
Part A
empirical formula CH₂, molar mass = 56.0 g/mol
Express your answer as a chemical formula.
– ΑΣΦ
?
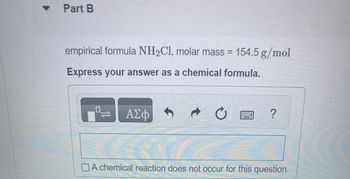
Transcribed Image Text:▾ Part B
empirical formula NH₂C1, molar mass = 154.5 g/mol
Express your answer as a chemical formula.
12
ΑΣΦ
t
O
?
A chemical reaction does not occur for this question.
Expert Solution
arrow_forward
Step 1: Molecular formula
Molecular formula can be defined as formula that represents total number of individual atoms present in a substance, We have been asked the molecular formula from given empirical formula.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use percent composition and molar mass to determine molecular formula. Analysis of a compound of carbon, hydrogen and oxygen showed that it is 52.13% C and 13.15% H, with O accounting for the remainder. In a separate experiment, the molar mass of the compound was found to be 46.08 g/mol. Determine the molecular formula of the compound. Enter the elements in the order C, H, O.arrow_forwardSample A 8.074 gram sample of an organic compound containing C, H and O is analyzed by combustion analysis and 20.88 grams of CO₂ and 4.274 grams of H₂O are produced. In a separate experiment, the molar mass is found to be 136.2 g/mol. Determine the empirical formula and the molecular formula of the organic compound. (Enter the elements in the order C, H, O.) Empirical formula: Molecular formula:arrow_forwardCertain antacids are composed of calcium carbonate, CaCO3. How many grams of calcium are present in a 400 g bottle of CaCO3 ? 235 g 160 g 0.160 g 9.99 garrow_forward
- Write the empirical formula for at least four ionic compounds that could be formed from the following ions:arrow_forwardComplete the table below for calculating the molar mass of the ionic compound chromium(II) sulfide. Formula Molar mass of ion Number of Mass of ion in one mole of ions chromium(II) sulfide Cation Cr2+ g/mol mol Anion S2. g/mol mol g Molar mass chromium(II) sulfide = g/molarrow_forwardHow many moles of nickel (II) cyanide are present in 4.61 grams of this compound? how many grams of nickle (II) cyanide are present in 4.61 grams of this compound?arrow_forward
- Combustion analysis of 1.200 g of an unknown compound containing carbon, hydrogen, and oxygen produced 2.086 g of CO2 and 1.134 g of H2O. What is the empirical formula of the compound?Combustion analysis of 1.200 g of an unknown compound containing carbon, hydrogen, and oxygen produced 2.086 g of CO2 and 1.134 g of H2O. What is the empirical formula of the compound?C2H5OC3H8O2C2H5O2C2H10O3arrow_forwardA certain compound has a percent by mass composition of 19.36% calcium, 34.25% chlorine and the rest is oxygen. What is the chemical formula of the compound? O Ca(CIO)2 O Ca(CIO4)2 O Ca(CIO2)2 O Ca(CIO3)2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY