
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%

Transcribed Image Text:) Listen
What is the molarity when 1.7 grams of potassium fluoride is dissolved to make 1.4
mL? Answer to 2 decimal places.
Your Answer:
Answer
units
Expert Solution
arrow_forward
Step 1
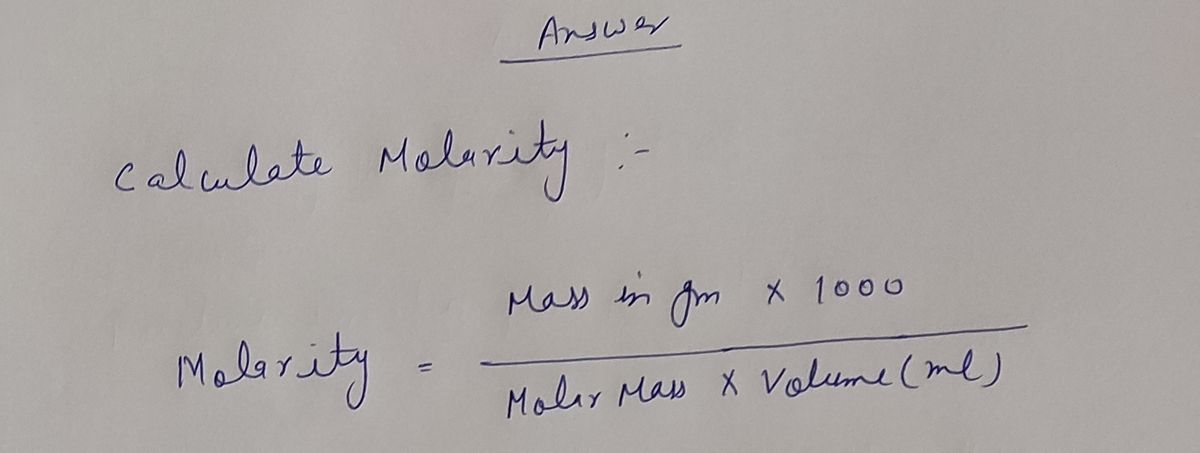
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 2arrow_forwardCan you explain this equation M(molarity)= moles of solute/liter of solution. COuld you do an example. So like if something is grams would you have to change it to moles?arrow_forwardDetermine the molarity (in mol/L) of nickel(II) sulfate when 2.78 g of nickel(II) sulfate is dissolved in enough water to produce 150 mL of solution. Report your answer to two decimal points in scientific notation.arrow_forward
- please answer question in second picture helparrow_forwardIf 2.49 g of CuNO3 is dissolved in water to make a 0.600 M solution, what is the volume of the solution in milliliters? volume: mLarrow_forwardHow many grams of CaCl2 (MM = 110.98 g/mol) are needed to make 475.0 mL of a 0.285 M solution? Your Answer: Answer unitsarrow_forward
- If 8.96 g of CUNO, is dissolved in water to make a 0.100 M solution, what is the volume of the solution in milliliters? volume: mLarrow_forwardWhat will be the final volume of water you need to add to 475 mL of a 2.8 M KCI solution to make 0.66 M solution? No decimal places in the answer. Your Answer: Answer unitsarrow_forwardBe sure to answer all parts. What is the molarity of a solution prepared using the given amount of solute and total volume of solution? a. 7.4 mol of KCl in 4.40 L of solution: b. 0.50 mol of NaNO3 in 603 mL of solution: Marrow_forward
- The molarity of 25% by mass HCI solution is?? The density of the HCI solution is 1.20 g/mL. Which is correct answer? a. 8.23 M b. 2.50 M c. 6.85 M d. 0.823 M e. 4.11 Marrow_forwardMass of NaCl __5.844______ gTotal volume of solution ___100______ mLMolarity of solution ____1_____ M (mol/L) Would the molarity of NaCl change if it had 1 gram of sugar in the solution as well? Explain your answer.arrow_forwardIf 5.42 g of CuNO, is dissolved in water to make a 0.410 M solution, what is the volume of the solution in milliliters? volume: mLarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY