
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
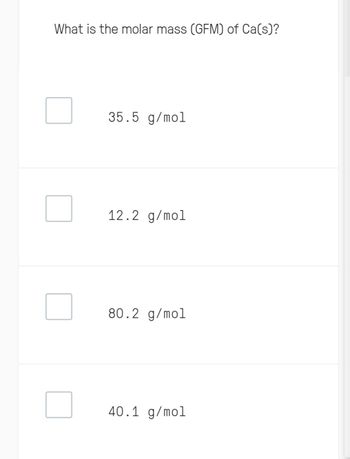
Transcribed Image Text:What is the molar mass (GFM) of Ca(s)?
35.5 g/mol
12.2 g/mol
80.2 g/mol
40.1 g/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the molar mass of H₂SO3? 82 g/mol 42 g/mol 113 g/mol 62 g/molarrow_forwardA major component of honey is fructose (C6H12O6) with a molar mass of 180.16 g/mol. A tablespoon (15.0 mL) of honey is considered one serving. How many tablespoons must be eaten to consume 0.1900 moles of fructose? Assume honey is 100% fructose. The density of honey is 1.45 g/mL.arrow_forwardSelect the number of moles of NaCl in 8.68 x 1025 sodium chloride formula units. O 144 mol NaCl O 8.68 x 1025 mol NaCl O 8.68 mol NaCl O 0.00694 mol NaClarrow_forward
- The molar mass of calcium phosphate, Ca3(PO4)2 is: Group of answer choices 278.18 g/mol 215.21 g/mol 230.19 g/mol 365.07 g/mol 310.18 g/molarrow_forwardWhich of the following options contain the highest mass? 1.0 mole Rb 1.0 mole K 1.0 mole Li 1.0 mole Naarrow_forwardComplete the table below for calculating the molar mass of the compound phosphorus triiodide. Molar mass of element Mass in one mole of phosphorus triiodide Moles g/mol mol %3D g g/mol x mol %3D Molar mass phosphorus triiodide = g/molarrow_forward
- A hydrocarbon compound (containing only H and C) is completely combusted in excess oxygen gas. If 0.440 g and 0.180 g of carbon dioxide (44.0 g/ mol) and water (18.0 g/mol) are created respectively, which formula below would be possible? C6H6 C2H4 C2H2 C2H6 C2H5arrow_forwardHow many moles of Cs are contained in 595 kg of Cs? 2.23 x 10² moles Cs 4.48 x 10³ moles Cs 7.91 x 104 moles Cs O 1.26 x 10³ moles Cs 5.39 x 10² moles Csarrow_forwardCalculate the Molarity of the solution given the following information. 25g KCI dissolved into 250 mL H20 1 mole KCI = 74.6 g %3Darrow_forward
- How many moles of fructose are in a sample containing 1.43 x 1024 molecules of fructose? 8.61 × 1047 mol O 1.16 x 10-48 mol O 2.38 x 10-1 mol O 2.37 mol O 0.420 molarrow_forwardWhat mass of AgI (234.8 g/mol) can be produced from a 0.4745 g sample that is 53.21% MgI2 (278.11 g/mol)? 0.2525 g 0.3460 g 0.4263 g 0.5901 garrow_forwardWhat is the Molar Mass or Molecular Weight for H2O? 20.0 g/mol O 18.02 g/mol O 2.02 g/mol O 19.0 g/molarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY