
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
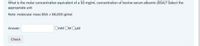
Transcribed Image Text:What is the molar concentration equivalent of a 50 mg/mL concentration of bovine serum albumin (BSA)? Select the
appropriate unit
Note: molecular mass BSA = 66,000 g/mol
Answer:
OmM OM OuM
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following reaction:H2CO3 + 2 NaOHNa2CO3 + 2 H2O(1) What is the equivalent mass of H2CO3? g/equiv(2) Calculate the number of equivalents in 27.1 grams of H2CO3. equivarrow_forwardPart A To what volume should you dilute 25 mL of a 11.0 MH₂SO4 solution to obtain a 0.160 M H₂SO4 solution? Express your answer using two significant figures. 195| ΑΣΦ V Submit Request Answer PMC ? Larrow_forwardnumber 15 (POST) please answer A&B ! thank you!arrow_forward
- What is the concentration in v/v% for 250. mL of solution contains 16 mL of acetone. (3 SF)arrow_forwardHow many nM are there in 3.4 µg/10 ml of NADH of molecular weight 664.44?arrow_forwardYou require 35g of solid NaOH, all you have is 4M solution, what volume of 4M should you use? Please explain, correct answer and typed answer only, (no copy paste)arrow_forward
- 22:11 Question 21 of 30 Submit Determine the amount in grams of KCI that exists in 20.3 g of a solution that contains 1.14 % KCl by mass STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 23.1 0.272 g KCI 6.022 x 1023 17.8 mol KCI 74.55 20.3 %m KCI 1.14 0.014 g solution 100 g КC/mol 1 1450 0.231 Tap here or pull up for additional resourcesarrow_forwardHow many grams of citric acid are contained in this 5.00 mL solution of citric acid if the molar mass of citric acid is 192 g/ mol? Group of answer choices 1.11 g 3.33 g 0.111 garrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY