
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
What is the mechanisms to get from the reactant to the products
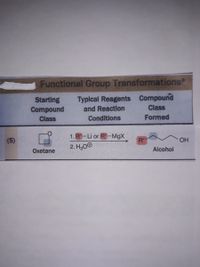
Transcribed Image Text:**Functional Group Transformations**
| Starting Compound Class | Typical Reagents and Reaction Conditions | Compound Class Formed |
|-------------------------|-----------------------------------------|------------------------|
| Oxetane | 1. R"–Li or R"–MgX | Alcohol |
| | 2. H₃O⁺ | |
**Description:**
This section outlines the transformation of the starting compound class, Oxetane, into the compound class known as Alcohol.
**Reagents and Conditions:**
1. The reaction involves organometallic reagents such as R"–Li (organolithium) or R"–MgX (Grignard reagent).
2. This is followed by the addition of an acid, specifically H₃O⁺ (hydronium ion).
The diagram shows the conversion process with structural representations of the compounds involved. The transformation results in the formation of an alcohol, which is indicated by the presence of the hydroxyl group (OH) in the product structure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Gives examples of reversible reactions that proceed very slowly but have an extremely high equilibrium constant. What can you say about its activation energy? How do we use or control the reaction? I know Sulfur trioxide and formation H2O but I need other examples.arrow_forwardA catalyst will... provide an alternate. pathway for a reaction that requires less activation energy be used up in the reaction provide an alternate. pathway for a reaction that requires more activation energyarrow_forwardUnderstanding and make them clear to differentiate between chemical kinetics and chemical equilibrium?arrow_forward
- Which of the following is not true of catalysts? O They become part of the final product. O They are unchanged by the reaction they catalyze. O They speed up reactions. O They do not change the normal position of a chemical equilibrium.arrow_forwardtab = -lock fn esc 14 → C Assignments-2022 Fall Term (1) X Acetylene and oxygen react to form carbon dioxide and water, like this: 2C₂H₂(g) +50₂(g) 4CO₂(g) + 2H₂O(g) O ADVANCED GENERAL CHEMISTRY Using Le Chatelier's Principle to predict the result of changing... Suppose a mixture of C₂H₂, O₂, CO₂ and H₂O has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation 4 Some C₂H₂ is removed. Some CO₂ is added. Explanation 1 46°F Mostly cloudy + https://www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IBMBkpcnaFu0F7UjsroJMKrbFHXPNvwILIE9xQia_u6lCg8... A "2 ? 1 Q A N @ - 2 Check ("* ("* W S X alt The pressure of O₂ will The pressure of CO₂ will H # ALEKS - Chisom Obidiegwu - Le X The pressure of C₂H₂ will ? The pressure of O, will ? 3 LU E change in composition D Q Search $ C by 4 R F LL ? 0…arrow_forwardExplain why high molecularity reactions are rare but not conflicting with the fact that increasing concentration increases the forward reaction rate.arrow_forward
- Because there is stability in reactants, A) before two reactants can form products, they must overcome an activation energy B) they can form products if one reactant loses some activation energy C) they can never form products, unless there in a huge input of energy D) they can never form productsarrow_forwardHow would the following MaxwellI Boltzmann distribution change if temperature were increased? Draw the expected change and explain what is happening to reaction rate. Number Of Molecules EA Energyarrow_forwardA reaction mechanism that has three steps will have how many reaction intermediates?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY