
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
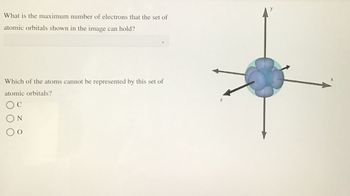
Transcribed Image Text:What is the maximum number of electrons that the set of
atomic orbitals shown in the image can hold?
Which of the atoms cannot be represented by this set of
atomic orbitals?
O C
N
Z
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3arrow_forwardDetermine the amount of energy lost by an electron that drops from the eight (n=8) to the first energy level (n=1) in a hydrogen atom. 2.15 x 10-18 J 2.08 x 10-19 J 2.97 x 10-20 J 9.14 x 10-18 Jarrow_forwardConsider the following three atomic orbitals. They are drawn to scale and orbital a has n=2. How many electrons can be placed in any orbital b? eTextbook and Media How many different sets of quantum numbers can describe an electron in orbital c? Arrange these orbitals (by letter only) in order of increasing stability for a multielectron atom. a 8 In a sodium atom, how many other orbitals have exactly the same energy as orbital b? Give the name of the element that has orbital a filled but orbitals b and c empty. Give the name of any common anion in which all orbitals of type c are filled. Assistance Used Give the names of the elements (ordered by atomic number and separated by a space) that have both an empty and partially filled orbital c.arrow_forward
- Working with gas discharge tubes in a spectroscopy lab, you measure the following set of lines for a gas comprised of a single element. 467.53 nm 319.60 nm 272.73 nm 250.57 nm 1. Would you be able to see these lines with ordinary húman vision? Which one(s) and why? 2. Knowing that the observed lines correspond to the final state of n = 3, identify the proton number Z for this element and thus reveal its identity. Hint: Calculate only as many lines as you need 3. Knowing the domain of validity of the Bohr model, determine the net charge of the atoms in your experiment.arrow_forwardWhich quantum number(s) determine the energy of an electron? Group of answer choices l and m1 n and l n and m1 ml only l onlyarrow_forwardLook at the following orbital diagram. What principles/rules does it break? Explain. 1s 2s 2parrow_forward
- What is the wavelength of light (in nm) emitted when an electron transitions fromn 3 to n = 2 in a hydrogen atom? Submit an answer to three signficant figures. nm 1 3 4 6. C 7 9. +/- x 100arrow_forwardA neutral atom has the following electron configuration: 1s²2s²2p 4 What is the chemical symbol for the 0 atom? 0 1 How many electrons does the atom have? How many 2s electrons are in the atom? 04 X 3 K Carrow_forwardplease solve question 1 and 2, thanks alot sirarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY