
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please set up the answer the correct way. I am having a hard time with putting the units in the correct spots.
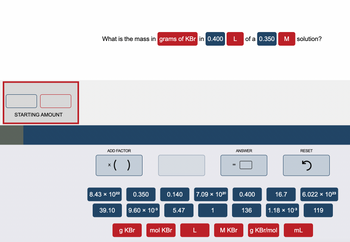
Transcribed Image Text:### Calculation of Mass in Grams of KBr for a Given Solution
#### Problem Statement:
What is the mass in **grams of KBr** in **0.400 L** of a **0.350 M** solution?
#### Input Section:
- **Starting Amount**: Input fields to enter the initial values. The left input field is for the initial volume in liters (L), and the right input field is for the initial molarity (M).
#### Calculation Section:
- **Add Factor**: Buttons to add factors to the calculation. For example, multiplication (x) and parentheses ( ).
- **Predefined Values**:
- 8.43 × 10²²
- 39.10
- 9.60 × 10^(-3)
- 5.47
- 0.350 (the molarity of the KBr solution)
- 0.140
- 7.09 × 10²⁰
- 16.7
- 1
- 136 (presumably the molar mass of KBr)
- 0.400 (the volume in liters)
- 1.18 × 10^(-3)
- 6.022 × 10²³ (Avogadro's number)
- 119
- **Units**:
- g KBr (grams of KBr)
- mol KBr (moles of KBr)
- L (liters)
- M KBr (molarity of KBr)
- g KBr/mol (grams per mole of KBr)
- mL (milliliters)
#### Output and Reset Section:
- **ANSWER**: Box to show the calculated answer.
- **RESET**: Button to reset the calculation.
### Example Calculation Steps:
1. **Calculate Moles of KBr**:
- Use the formula \( \text{Moles} = \text{Molarity} \times \text{Volume} \)
- Here, \( \text{Moles} = 0.350 \, M \times 0.400 \, L \)
- \( \text{Moles} = 0.140 \, \text{mol KBr} \)
2. **Convert Moles to Grams**:
- Use the molar mass of KBr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Trial 2 (Table 4) Slope _______________________ Reciprocal Slope ________________arrow_forwardReferring to your best straight line graph, what are the units of the slope?What physical property of steel is the slope of this line related to? Use the graphed data and your best trendline equation to predict the diameters of the three unknown ball bearings.Show your calculations below and report your three results in the data table above with 2 decimal place accuracy. ] 7. How could we improve the accuracy of the largest unknown ball bearing's diameter prediction? can you help me answer all this questions please, ai really appreciated it.arrow_forwardSerious injuries can occur when students attempt to force the pipet bulb onto the top of the pipet. The blue bulbs we use in lab are not meant to attach to the pipet, they simply rest on the top. Which of the following items will help you be more successful when working with a pipet? Group of answer choices Gently place the pipet bulb on the pipet Keep the tip of the pipet submerged when filling It is best to fill the pipet to a point just above the calibration line, then adjust the volume by slowly releasing liquid. Liquid should fully fill the pipet bulb The more aggressive you are with the pipet bulb, the quicker you will get out of labarrow_forward
- I have calculated this problem various times, but every time I input the answer into the assignment it says it's wrong, I can't find the issue. What could it be?arrow_forwardWhy should repetitive measurements always be made, if possible, when performing an experiment. (Please give a detailed answer, thank you)arrow_forwardCa you please answer this in 10 minutes? pretty pleaaseee. Thank you ?arrow_forward
- Make a dilution series to get to 1:100. Start with making 1:10 dilution by adding 1mL of sample and 9mL of diluent, now continue the series to get to 1:100. The formula you may use is V1D1=V2D2arrow_forwardFor liquid B, you decide to use a 50 mL burette to once again dispense 5.00 mL of liquid B into a convenient container. You find that the empty container has a mass as 3.05 grams. After obtaining the mass of the empty container you place it beneath the burette and open the stopcock valve. Although you try to stop the flow of liquid B at exactly the 5.00 mL mark you overshoot slightly and end up dispensing 5.14 mL. What is the mass of liquid-B that was dispensed if you continue with the density determination for liquid B and find that the mass of the container plus the 5.14-mL of liquid B is 7.63-grams?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY