
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
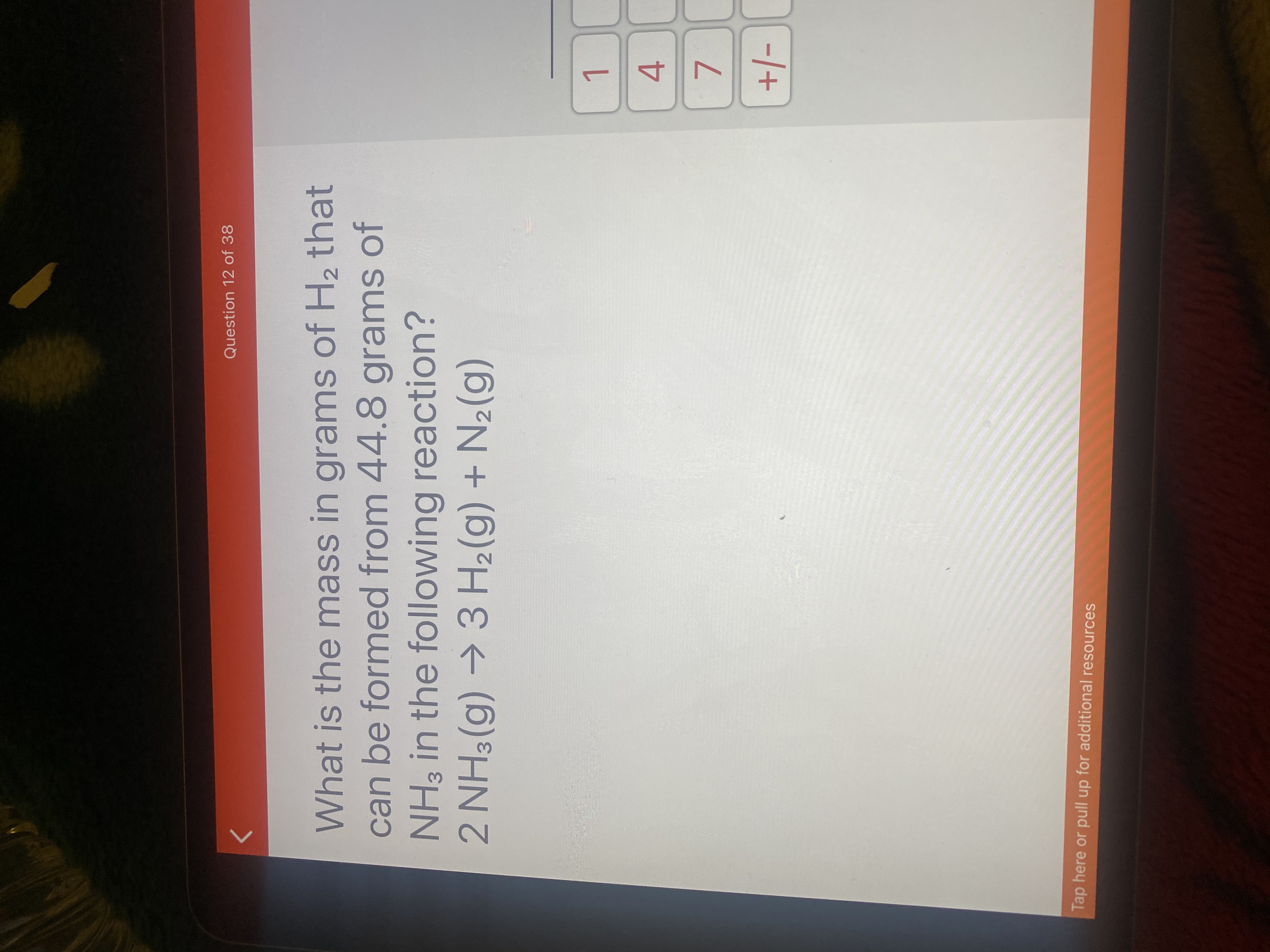
Transcribed Image Text:What is the mass in grams of H2 that
can be formed from 44.8 grams of
NH3 in the following reaction?
2 NH3 (g) →3 H2(g) + N2(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 0.300 g of Fe3O4 reacts with excess O₂ to give Fe₂O3 in 26.0% yield according to the following balanced equation. 4Fe3O4(s) + O₂(g) 6Fe₂O3(s) → Calculate the theoretical yield of Fe₂O3. g Calculate the actual yield of Fe2O3. garrow_forwardBased on the balanced reaction: 2 Al + 3 Cl2→ 2 AlCl3, what is the maximum number of moles of AlCl3 that could be made by the reaction of (2.11x10^1) mol of Al and (1.990x10^1) mol of Cl2?arrow_forwardCalculate the quantity of O2 would be required to generate 13.0 mol of NO2 in the reaction below assuming the reaction has only 66.5% yield. 2 NO (g) + O2 (g)→2 NO2 (g)arrow_forward
- What is the mass in grams of phosphorus trichloride that can be formed from 232.6 grams of chlorine gas based on the balanced chemical equation? balanced chemical equation: P4(s) + 6 Cl2(g)-> 4PCl2(g)arrow_forwardWhat is the mass in grams of NO that will be produced from 33.5 g of NO₂ reacted with excess water in the following chemical reaction? 3 NO₂(g) + H₂O(l) → 2 HNO₃(g) + NO(g)arrow_forwardWhat is the mass in grams of CO₂ that can be produced from the combustion of 2.45 moles of butane according to this equation: 2 C₄H₁₀(g) + 13 O₂(g) → 8 CO₂(g) + 10 H₂O(g)arrow_forward
- According to the following balanced equation, what mass (in g) of MgO can be made from 7.5 g of 02? 2Mg (s) + O2 (g) 2MGO (s)arrow_forwardWhat is the mass in grams of CO₂ that can be produced from the combustion of 6.54 moles of butane according to this equation: 2 C₄H₁₀(g) + 13 O₂(g) → 8 CO₂(g) + 10 H₂O(g)arrow_forwardWhat is the mass in grams of NO that will be produced from 37.9 g of NO2 reacted with excess water in the following chemical reaction? 3 NO2(g) + H2O(1) → 2 HNO3(g) + NO(g)arrow_forward
- How many grams of NO will be produced from 80.0 g of NO₂ reacted with excess 2 water in the following chemical reaction? 3 NO₂(g) + H₂O(l) → 2 HNO₂(g) + NO(g) A) 17.4 g B) 157 g C) 52.2 g D) 40.9 garrow_forwardCalculate the theoretical yield (in grams) and percent yield in the following situations: Consider the following reaction:CO + 2 H2→CH3OH What is the theoretical yield and percent yield of CH3OH if the reaction of 2.50 g of H2with an excess of CO produces 8.00 g ofCH3OH?arrow_forwardEach step in the following process has a yield of 80.0%.CH4+4Cl2⟶CCl4+4HClCCl4+2HF⟶CCl2F2+2HClThe CCl4 formed in the first step is used as a reactant in the second step. If 2.00 mol CH4 reacts, what is the total amount of HCl produced? Assume that Cl2 and HF are present in excess.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY