
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
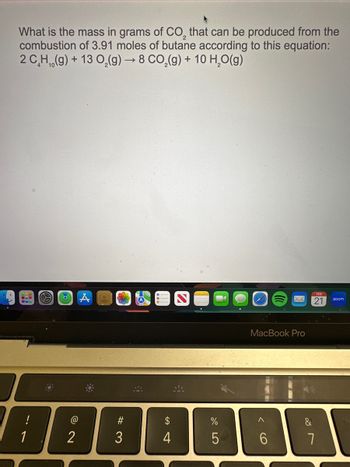
Transcribed Image Text:What is the mass in grams of CO, that can be produced from the
combustion of 3.91 moles of butane according to this equation:
2 C_H,,(g) + 13 O,(g) — 8 CO,(g) + 10 H,O(g)
1
O
@
2
**
#
3
$
4
%
5
MacBook Pro
H
6
&
21
7
zoom
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Small quantities of oxygen can be prepared in the laboratory by heating potassium chlorate, KC1O3(s). The equation for the reaction is 2 KC1O₂ → 2 KCl +30₂ Calculate how many grams of O₂(g) can be produced from heating 73.9 g KClO3(s). mass: g 02arrow_forwardWhat is the mass in grams of NO that can be formed from 4.96 moles of NO, in the reaction below? 3 NO2 (g) + H,O (g) → 2 HNO3 (g) + NO (g) ->arrow_forwardSmall quantities of oxygen can be prepared in the laboratory by heating potassium chlorate, KClO₂ (s). 3 The equation for the reaction is 2 KCIO3 → 2 KCl + 30₂ Calculate how many grams of O₂(g) can be produced from heating 23.3 g KClO₂ (s). 3 mass: g 0₂arrow_forward
- 3 Cu +8HNO3 --> 3 Cu(NO3)2 2 NO + 4 H₂O In the above equation how many grams of water can be made when 6 grams of HNO3 are consumed? Round your answer to the nearest tenth. If you answer is a whole number like 4, report the answer as 4.0 Use the following molar masses. If you do not use these masses, the computer will mark your answer incorrect.: Molar Element Mass Hydrogen 1 Nitrogen 14 Copper 63.5 Oxygen 16arrow_forwardGaseous hydrogen and oxygen can be prepared in the laboratory from the decomposition of gaseous water. The equation for the reaction is 2H,O(g)2H, (g) +0,(g) Calculate how many grams of O, (g) can be produced from 14.6 g H, O(g). gO2 mass:arrow_forwardConsider the following equation: Al4C3 + 12 H2O → 3 CH4 + 4 Al(OH)3 How many grams of Al(OH)3 will be produced when 0.986 mol of CH4 is formed?arrow_forward
- Determine the mass in grams of CO₂ that is produced by the complete reaction of 10.49 grams of C₆H₁₂ (cyclohexane) according to the following combustion reaction: C₆H₁₂(l) + 9 O₂(g) → 6 CO₂(g) + 6 H₂O(g)arrow_forwardAccording to the following reaction, how many grams of chlorine gas are necessary to form 0.672 moles phosphorus trichloride? PA(s) + 6C12 (g) → 4PCI3 (t) chlorine gasarrow_forwardHow many moles of NH, can be produced from 2.61 moles of nitrogen in the following reaction: N, (g) + 3 H, (g) → 2 NH, (g)arrow_forward
- "You begin with 21.2 grams of CO(g) and 9.91 grams of O2(g) . If 17.48 grams of CO2(g) are produced, what is the percent yield? 2 CO(g)+ 1 O2(g) → 2 CO2(g)"arrow_forwardDetermine the number of grams of C₄H₁₀ that are required to completely react to produce 8.70 mol of CO₂ according to the following combustion reaction: 2 C₄H₁₀(g) + 13 O₂(g) → 8 CO₂(g) + 10 H₂O(g)arrow_forwardSmall quantities of oxygen can be prepared in the laboratory by heating potassium chlorate, KClO3(s). The equation for the reaction is 2KClO3⟶2KCl+3O2 Calculate how many grams of O2(g) can be produced from heating 12.2 g KClO3(s).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY