
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Question 2 of 30
Submit
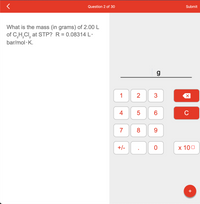
What is the mass (in grams) of 2.00 L
of C,H,CI, at STP? R = 0.08314 L·
2* 2
2.
bar/mol · K.
1
4
6
C
7
9.
+/-
х 100
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The Haber Process synthesizes ammonia at elevated temperatures and pressures. Suppose you combine 1580 L of nitrogen gas and 4590 L of hydrogen gas at STP, heat the mixture to run the reaction, then isolate the ammonia from the reaction mixture. What volume of NH₃ , measured at STP, would be produced? Assume the reaction goes to completion. N₂ (g) + 3 H₂ (g) → 2 NH₃ (g)arrow_forwardFind the pressure in Torr of 0.0080 mol of an ideal gas contained in 125 L at 15 °C: R = 62.36 L·Torr/(mol·K)arrow_forwardThe partial pressure of water vapor in saturated air at 34 °C is 5.25×10-2 atm. (a) How many molecules of water are in 1.16 cm3 of saturated air at 34 °C? (b) What volume of saturated air at 34 °C contains 0.563 mol of water?arrow_forward
- Mass of a vacuumed (empty) vessel of volume 5 L is 640.05 g, and mass of the same vessel filled with this hydrocarbon up to the pressure 2.0 atm at 22oC is 658.23 g. What is the molecular formula of the hydrocarbon?arrow_forwardA flask at room temperature contains equal numbers of di-nitrogen molecules and krypton atoms. (a) Which of the two gases exerts the higher partial pressure? (b) Which gas has a higher kinetic energy per molecule/atom? (c) Which gas has molecules with a higher velocity? Explain your answers.arrow_forwardA flask of volume 0.753 L contains a sample of chlorine gas, Cl2, with a pressure of 198 Torr at 25.8 degrees C What mass of sodium, in grams, is required to completely react with the chlorine to make sodium chloride? 2 Na (s) + Cl (g) - - > 2 Na C1 (s)arrow_forward
- A sample of 3.42 mol of xenon is confined at low pressure in a volume at a temperature of 86 °C. Describe quantitatively the effects of each of the following changes on the pressure, the average kinetic energy per molecule in the gas, and the root-mean-square speed. (a) The temperature is increased to 199 °C (b) The volume is tripled. (c) The amount of xenon is decreased to 1.87 mol Give each answer as a decimal factor of the form: new value factor old value. A factor of 1 means no change. ChangeP KEavgmsarrow_forwardFor the reaction A + B --> 2 C, Kç = 0.16. A 2.00 L flask is filled with 1.00 mol A and 1.00 mol B, and allowed to come to equilibrium. Determine the concentrations, in moles/liter, of A, B and C at equilibrium. (Do not input units, 3 sig figs). A = B = C =arrow_forwardA sample of H2(g) was collected over water in a volume of 12.0 mL at 18 °C under atmospheric pressure of 770. Torr. Partial pressure of water is P(H2O, 18 °C) = 16 Torr. Find the mol’s of H2(g) in the samplearrow_forward
- Calculate the total pressure (in atm) of a mixture of 3.00 x 10-2 mol of helium, He, and 7.00 x 10- mol of oxygen, O2, in a 6.00 L flask at 20.°C. Assume ideal gas behavior. Total pressure atm ||arrow_forwardWhat is the pressure of a mixture of 0.0500 g of H2, 1.00 g of N2, and 1.60 g of Ar in a container with a volume of 4.00 L at 20 °C?arrow_forwardlithium reacts with H2O to produce hydrogen.(a) Write a balanced chemical equation for the reaction, using the smallest integer coefficients possible. Write states in brackets. (Hint: LiOH is the second product.) (b) Calculate the mass of pure Li that will furnish 16.3 L of hydrogen at a pressure of 0.860 atm and a temperature of 30.4°C.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY