
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
9
Don't copy paste ans please
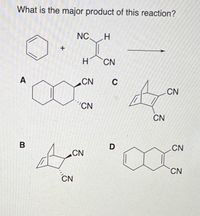
Transcribed Image Text:What is the major product of this reaction?
NC H
H
CN
A
CN
C
CN
"CN
CN
B
D
CN
CN
CN
CN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- me File Edit View History Bookmarks Profiles Tab Window Help Watch Gilmorex * Dementia Frien x 1 unread) - dte X Lobby Top Hat X 2 Dashboard xV Lives Well Livec x A ALEKS - David x Psychology Re i www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IVDWKW_BBZZ6tTytly4Fcfu6zOtOf8oMM9s8f0Bcp47VrzRTjh-0_CAFLD-fp-V... Spotify Web Playe.. M Common Ethical D.. O CHEMICAL REACTIONS Using a chemical equation to find moles of product from moles ... Dav Gaseous ammonia chemically reacts with oxygen (0,) gas to produce nitrogen monoxide gas and water vapor. Calculate the moles of water produced by the reaction of 0.700 mol of oxygen. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. Explanation Check © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardA ALEKS - Dona Luc - Lean H My Grades - 2021 Spring Term (2 X Tutor.com Learning Suite M Hey - lucdona7@gmail.com - Gm x www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-IBIZZhveDw7yX8A9043nt5P1XWJwAREDsbwIERg1UdvpRqH651Jk. O MATTER Solving applied density problems Mr. Auric Goldfinger, criminal mastermind, intends to smuggle several tons of gold across international borders by disguising it as lumps of iron ore. He commands his engineer minions to form the gold into little spheres with a diameter of exactly 6 cm and paint them black. However, his chief engineer points out that customs officials will surely notice the unusual weight of the "iron 3. ore" if the balls are made of solid gold (density 19.3 g/cm). He suggests forming the gold into hollow balls 3. instead (see sketch at right), so that the fake "iron ore" has the same density as real iron ore (5.15 g/cm). One of the balls of fake "iron ore," sliced in half. Calculate the required thickness of the walls of each hollow…arrow_forwarde File Edit View History Bookmarks Profiles Tab Window Help Watch Gilmore Girls x * Dementia Friend C (2 unread) - dt882 x Lobby | Top Hat A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IvdWKW_BBZZI6tTytly4Fcfu6zOtOfE Spotify Web Playe... M common Ethical D.. O CHEMICAL REACTIONS Interconverting number of atoms and mass of compound Calculate the number of carbon atoms in a 150.0 g sample of camphor (C10H0). Be sure your answer has a unit symbol if necessary, and round it to 4 significant digits. x10 Explanation Check MAR 13arrow_forward
- Need help with #2arrow_forwardLab Exam 1- Requires Respon X 021 Summary - CHM151-N803: Ger X A ALEKS - Delana Bailey - Learn X C Sign In or Sign Up | Chegg.com X www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IJgg7xIASweFMYphv4tCLy6BMpSgONHO-bYrHeBZ4S54EqYg5AJLeyt82s1i-_fz32exQQdKy96vMC... ☆ → C tab caps lock shift O CHEMICAL REACTIONS Solving for a reactant using a chemical equation Ammonium phosphate ((NH4),PO4) What mass of ammonium phosphate is produced by the reaction of 7.4 g of ammonia? Round your answer to 2 significant digits. esc Explanation ! 1 Q e A 1 control option @ 2 N Check W S #3 is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H₂PO4) with ammonia (NH₂). X H command E D X $ 4 C R FL do 5 % V T MacBook Pro 6 Y & 7 G H B Ⓒ2022 McGraw Hill LLC. All Rights Reserved. U 8 J | ΤΝΤ Μ N Nitrogen N2 gas and hydrogen X + ( 9 K 0 ) 0 Terms of Use | Privacy Center | Accessibility L 0/5 P , | 1 command option WE { [ + 11 Delana 2 | 000 Ar KI } ? D Update…arrow_forwardHelp 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forward
- Pls answer all 7 questions. I really need it and I don't understandarrow_forwardUsing the following monomer: H2C CH C CH- CH CH CH C-F (the benzene ring does not react) draw the mechanism for the formation of the polymer, stopping after attaching three units of the monomer together. DETAIL!! You will need more than one reaction, and yes, this will be one time you need to consider the mechanism. (20 pt.)arrow_forwarde File History Bookmarks Profiles Tab Window Help Edit View 14 unread) - dt x Lobby | Top Hat x O ALEKS x A ALEKS - Reyna Watch Gilmore X * Dementia Frien x i www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IVDWKW_BBZZ16tTytly4Fcfu6zOtOf8oMM9s7G0XWajIKntF38TIGB Spotify Web Playe.. M Common Ethical D.. O THERMOCHEMISTRY Understanding the definitions of heat and work A mixture of gaseous reactants is put into a cylinder, where a chemical reaction turns them into gaseous products. The cylinder has a piston that moves in or out, as necessary, to keep a constant pressure on the mixture of 1 atm. The cylinder is also submerged in a large insulated water bath. (See sketch at right.) From previous experiments, this chemical reaction is known to release 141. kJ of energy. The position of the piston is monitored, and it is determined from this data that the piston does 358. kJ of work on the system during the reaction. O exothermic Is the reaction exothermic or endothermic? O…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY