
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
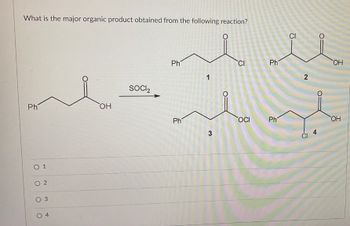
Transcribed Image Text:What is the major organic product obtained from the following reaction?
Ph
OH
○ 1
02
03
SOCI₂
Ph
CI
CI
Ph
OH
1
2
Ph
OCI
Ph
OH
3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- The structure of benzene-1,2,3-tricarboxylic acid (pKa1 =2.88, pKa2 4.76, pKa3 = 7.13) can be seen on the right. What is the predominate form of benzene-1,2,3-tricarboxylic acid in a solution that has a pH of 6.0?arrow_forwardWhat is the percent dissociation of acetic acid if the solution has a pH = 4.74 and a pK a = 4.74? What is the percent dissociation of acetic acid if the solution has a pH = 4.74 and a pK a = 4.74? 100% 10% 1% 50%arrow_forwardWhat will happen to this equilibrium when NaOH is added? NH3 + H+ NH4+arrow_forward
- H3PO4 is a triprotic acid with the following acid dissociation constants: H3PO4 ⇌ H2PO4- + H+ Ka1 = 1.2 × 10-4 H2PO4- ⇌ HPO42- + H+ Ka2 = 4.3 × 10-9 HPO42- ⇌ PO43- + H+ Ka3 = 7.2 × 10-12 What is K for the following equilibrium: PO43- + 3H+ ⇌ H3PO4arrow_forwardl(OH)3 has an OH-1 in the formula so we don't use water in the reaction because it just cancels out Al(OH)3 + H2O ⇔ Al+3 + 3 OH-1 + H2O Answer each of the following for the following reaction Al(OH)3 ⇔ Al+3 + 3 OH-1 Al(OH)3 has an OH-1 in the formula so is treated like a Blank 1. Fill in the blank, read surrounding text. base and we should use a one-sided arrow There is no acid or conjugate acidarrow_forwardWhat is the product of the following reaction? aq. HCI CN .CO2H NH2 II CO2 K II IVarrow_forward
- Calculate the pOH of a substance with an H+ concentration of 10-4 10 O -4 O -10arrow_forwardAnswer the following questions about acid-base equilibrium reactions (d)Based on the following Kavalues, which is the strongest acid and which is the weakest acid? Ka (HNO2) = 4.5 x 10 –4Ka (H2SO3) = 1.5 x 10 –2Ka (H2CO3) = 4.3 x 10 –7arrow_forwardIdentify the weaker base and the correct relative value of the equilibrium constant, K, for this reaction. OH O I Il is the weaker base; K 1 Ill is the weaker base; K 1 + II III OH IVarrow_forward
- Given with the Kp of the different bases below, which of the corresponding conjugate acids is the strongest acid? Base Kb CIO- 3.3 x 10-7 CO3-2 1.8 x 10-4 HS- 1.8 x 10-7 NH2CH3 4.4 x 10-4 HC10 HCO3- H2S NH3CH3+arrow_forwardWhat is the structure of the conjugate acid for the reaction shown below? NH OK NH3 C OA Oc D 00arrow_forwardConsider the acid-base reaction below: (Question 14) Ca(HCO3)2 + Ca(OH)2 ----> Compound A + H2O (OR Ca(HCO3)2 + Ca left parenthesis OH right parenthesis 2 ----> Compound A + H2O) One of the single ions that is used to form compound A is ____. Assume enough of each compound is used to get a complete reaction. Question 14 answer choice options: C32- (OR C3 2 minus) C43- (OR C4 3 minus) C3- (OR C 3 minus) CO- (OR C O 1 minus) CO2- (OR C O 2 minus) CO43- (OR C O4 3 minus) CO4- (OR C O4 1 minus) C22- (OR C2 2 minus) CO32- (OR C O3 2 minus) C4- (OR C4 1 minus) C2- (OR C 2 minus) C33- (OR C3 3 minus) CO6- (OR C O 6 minus) C23- (OR C2 3 minus) C2- (OR C2 1 minus) C- (OR C 1 minus) C3- (OR C3 1 minus)…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY