
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
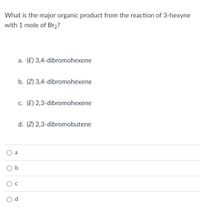
Transcribed Image Text:What is the major organic product from the reaction of 3-hexyne
with 1 mole of Br2?
a. (E) 3,4-dibromohexene
b. (Z) 3,4-dibromohexene
c. (E) 2,3-dibromohexene
d. (Z) 2,3-dibromobutene
a
b
O c
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Choose the two alkyl bromides and the hydrogenation method that must be used to synthesize these alkenes from acetylene. Enter your answer as two letters in alphabetical order, followed by a number; i.e. ac2, not ca2. Do not use punctuation. Alkyl Halides a. CH3CH,Br. b. CH3(CH2)4Br c. CH3(CH2);Br d. CH3(CH2)6Br e. CH3(CH2);Br f. CH3(CH2);Br g. CH3(CH2)12B Hydrogenation Method 1. H2, Lindlar's catalyst 2. Na, NH3 (1) Alkene #1: H3C CH3 Alkene #2: CH3 H3Carrow_forwardC. D. B. 1) Give the major organic product of the following reaction. Don't forget stereochemistry in certain cases. 2) Assign the reaction as addition, elimination, substitution, or rearrangement. A. D D 1. H₂O/Hg(OAC) 2 2. NaBH4 Br₂ CCl4 (inert solvent) H₂/Pd/C HBr Type of Reaction: Type of Reaction: Type of Reaction: Type of Reaction:arrow_forwardG.255.arrow_forward
- helparrow_forwardWhat is the IUPAC name of the following compound? CH, a. (R)-4-methyl-2-hexyne b. (S)-4-methyl-2-hexyne c. (R)-3-methyl-4-hexyne d. (S)-3-methyl-4-hexyne aarrow_forwardWhich of the following molecules has the highest heat of hydrogenation? A. 2,3-dimethylhex-1-ene B. 2,3-dimethylhex-2-ene C. (E)-2,3-dimethylhex-3-ene D. 4,5-dimethylhex-1-ene Aarrow_forward
- 3. Draw out 3-methylpentane. Use lower-case letters to label each TYPE of hydrogen (i.e., those that would lead to a different product) on each compound.arrow_forwardHow would you prepare ethyl isopropyl ether?a.) Reaction of ethoxide anion with 2-chloropropaneb.) Reaction of isopropoxide anion with chloroethanec.) Reaction of isopropoxide anion with 2-chloropropaned.) Reaction of ethoxide anion with chloroethanearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY