
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What is the Lewis dot structure, # of electron clouds, Hybridization about X, Electron Geometry, Molecular Geometry, and 3-D model of C6H6 (benzene).
Is Benzene polar or nonpolar?
Show bond angles and answer each question for each central atoms if applicable.
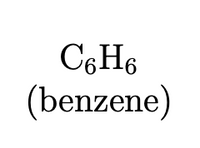
Transcribed Image Text:C6H6
(benzene)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the molecular model kit to construct the following models, and then fill in the boxes for each molecule. When possible. show a resonance Lewis structure. Use formal charge, when possible to select the best structure. Follow example for CH4 below. Formula #VES Lewis Structure H CH4 8 H C H -I H Resonance? (Y/N) Show N Electron Group & Bond Angle Tetrahedral 109° Molecular Geometry Polar? (Y/N) tetrahedral N VSPER Sketch H 1 H 109.5° H H Hybridization sp³arrow_forwardUse the molecular model kit to construct the following models, and then fill in the boxes for each molecule. When possible. show a resonance Lewis structure. Use formal charge, when possible to select the best structure. Follow example for CH4 below. Formula #VES CH4 8 NH3 H₂O CO₂ Lewis Structure H H-C-H H Resonance? (Y/N) Show N Electron Group & Bond Angle Tetrahedral 109° Molecular Polar? Geometry (Y/N) tetrahedral N VSPER Sketch H H 1 109.5° H H Hybridization sp³arrow_forward5. |- The molecule pictured below is acrylonitrile, the building block of the synthetic fiber Orlon. No explanations required H H H-c=C-c=N: a) Which is the longer carboncarbon bond? b) Which is the stronger carbonncarbon bond? c) Which is the most polar bond? Explain. Indicate the polarity of this bond by writing the partial charges and drawing its dipole moment.arrow_forward
- ructions tiple Attempts Not allowed. This test can only be taken once. ce Completion This test can be saved and resumed later. Your answers are saved automatically. Jestion Completion Status: 1 2 3 4 QUESTION 1 Enter the number of valence electrons and number of possible bonds for each of the following element CARBON: Valence electrons: 4 ; Bonds needed: 4 OXYGEN: Valence electrons: 6 ; Bonds needed: 2 HYDROGEN: Valence electrons: 1 NITROGEN: Valence electrons: 5 SULFUR: Valence electrons: PHOSPHORUS: Valence electrons: CHLORINE: Valence electrons: FLUORINE: Valence electrons: I For Blank 9 ; Bonds needed: 1 ; Bonds needed: 3 ; Bonds needed: ; Bonds needed: ; Bonds needed: ; Bonds needed: Click Save and Submit to save and submit. Click Save All Answers to suve all answers,arrow_forwardFind the hybrid orbital type for each atom, the carbon-carbon-oxygen bond angle, the molecular geometry of oxygen, and the number of sigma and pi bonds in the molecule.arrow_forwardDraw the Lewis structure for NH3 . Is the molecule polar or nonpolar? What is the electronic geometry, molecular geometry, and hybridization of nitrogen? Lastly, draw the 3-D structure of the entire molecule.arrow_forward
- i wanna help to complete this table (lewis structure, molecular shap and polar or non polar) table in attachments. thanks you so mucharrow_forwardDraw out the 3 dimensional VSEPR geometry for the compound. In the video start with a blank page and show all steps necessary to obtain the VSEPR geometry structure with proper bond angles. To answer the question in this quiz, provide the total valence electron count. Useful information: X (7 valence electrons), A (6 valence electrons) Chemical formula: AX2arrow_forwardUse the molecular model kit to construct the following models, and then fill in the boxes for each molecule. When possible. show a resonance Lewis structure. Use formal charge, when possible to select the best structure. Follow example for CH4 below. Formula #VES Lewis Structure H CH4 8 H C H -I H Resonance? (Y/N) Show N Electron Group & Bond Angle Tetrahedral 109° Molecular Geometry Polar? (Y/N) tetrahedral N VSPER Sketch H 1 H 109.5° H H Hybridization sp³arrow_forward
- Question 1: Draw the Lewis structure AND the 3D structure of the following molecules/ions, specifying the boundary forms of resonance and the resonance hybrid if applicable. Indicate whether the molecule (or ion) is polar or not. d) XeSI4 e) CH2CHCH2COOH f) CH3CCCH2CH3arrow_forwardplease check the screenshot fiind Lewis Structure (show all electrons and bonds) e– geometry Approx. Bond angles Polar or non-polar (don't worry about ions) Total lone pairs in the entire molecule Number of nonbonding electron pairs on the central atomarrow_forwardDecide whether the Lewis structure proposed for each molecule is reasonable or not. proposed Lewis structure Is this a reasonable structure? If not, why not? molecule O Yes, it's a reasonable structure. O No, the total number of valence electrons is wrong. NH, H N=H The correct number is:| No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: LI Yes, it's a reasonable structure. :F: O No, the total number of valence electrons is wrong. BF, The correct number is: :F-B –F: O No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: U Yes, it's a reasonable structure. F No, the total number of valence electrons is wrong. The correct number is: IF No, some atoms have the wrong number of electrons around them. .. F: The symbols of the problem atoms are: U * If two or more atoms have the wrong number of valence electrons around them, just enter the chemical symbol for the atom as many times as…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY