
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
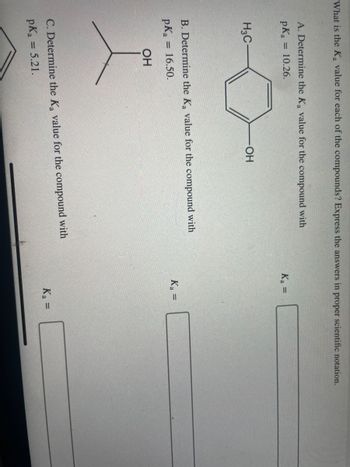
Transcribed Image Text:What is the Ka value for each of the compounds? Express the answers in proper scientific notation.
A. Determine the Ka value for the compound with
pKa = 10.26.
H3C-
OH
B. Determine the K₂ value for the compound with
pK₂ = 16.50.
OH
C. Determine the K₂ value for the compound with
pKa
= 5.21.
K₁ =
K₂ =
K₁ =
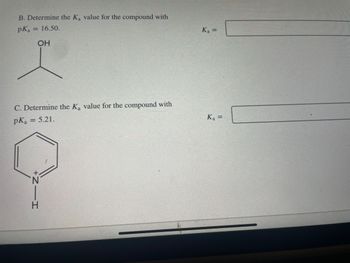
Transcribed Image Text:B. Determine the Ka value for the compound with
pKa = 16.50.
OH
C. Determine the K₂ value for the compound with
pK₂ = 5.21.
K₁ =
K₁ =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the Lewis dot structure for each of the following molecular compounds. CHC13 Select Draw Templates More C H Cl Erase ✪ 2 Q NHF, Select Draw Templates More N H F Q 2arrow_forwardPlease don't provide handwriting solutionarrow_forwardSample #1 has an absorbance of 0.300. Sample #2 is the same substance and has an absorbance of 0.600 using the same equipment. What is the relative concentration of the second sample? Select one: a. The concentration of sample #2 is the square root of that of #1. b. The concentration of sample #2 is that of sample #1 squared. c. The concentration of sample #2 is half that of sample #1. d. The concentration of sample #2 is twice that of sample #1.arrow_forward
- c. Explain the difference of pka of the following two compounds Overcas trans are pka H HADICH 35 Η Н han cis 43 I I H Ye more stable than 1 XXarrow_forwardType Formula Ksp Bromides PbBr2 6.3 × 10-6 AgBr 3.3 × 10-13 Carbonates BaCO3 8.1 × 10-9 CaCO3 3.8 × 10-9 CoCO3 8.0 × 10-13 CuCO3 2.5 × 10-10 FeCO3 3.5 × 10-11 PbCO3 1.5 × 10-13 MgCO3 4.0 × 10-5 MnCO3 1.8 × 10-11 NiCO3 6.6 × 10-9 Ag2CO3 8.1 × 10-12 ZnCO3 1.5 × 10-11 Chlorides PbCl2 1.7 × 10-5 AgCl 1.8 × 10-10 Chromates BaCrO4 2.0 × 10-10 CaCrO4 7.1 × 10-4 PbCrO4 1.8 × 10-14 Ag2CrO4 9.0 × 10-12 Cyanides Ni(CN)2 3.0 × 10-23 AgCN 1.2 × 10-16 Zn(CN)2 8.0 × 10-12 Fluorides BaF2 1.7 × 10-6 CaF2 3.9 × 10-11 PbF2 3.7 × 10-8 MgF2 6.4 × 10-9 Hydroxides AgOH 2.0 × 10-8 Al(OH)3 1.9 × 10-33 Ca(OH)2 7.9 × 10-6 Cr(OH)3 6.7 × 10-31 Co(OH)2 2.5 × 10-16 Cu(OH)2 1.6 × 10-19 Fe(OH)2 7.9 × 10-15 Fe(OH)3 6.3 × 10-38 Pb(OH)2 2.8 × 10-16 Mg(OH)2 1.5 × 10-11 Mn(OH)2 4.6 × 10-14 Ni(OH)2 2.8 × 10-16 Zn(OH)2 4.5 × 10-17 Iodides PbI2 8.7 × 10-9 AgI 1.5 × 10-16 Oxalates BaC2O4 1.1 × 10-7 CaC2O4…arrow_forward9. Assume the exact mass for compound X is 151.9472. a. What is the molecular formula, b. What is the index of hydrogen deficiency. 73 100 90 80- 70- 60- 50 40에 152 30- 20- 107 135 55 10- 26 18 0- 10 150 160 170 20 30 40 50 60 70 80 90 100 110 120 130 140 30- 25- 20- 15- 10어 3500 3000 2500 2000 1500 1000 Wavenumber (cm-1) c. Draw 2 structures that are consistent with the data -812 914 -1137 1265 --1342 -1395 1432 1717 -2571 -2670 3067 Transmittancearrow_forward
- Balance each sidearrow_forwardB 50ng/ml C500 ng/mlarrow_forwardFor numbers 24-25: A solution contains NaHCO3, Na2CO3, and NaOH, either alone or in a permissible combination. Titration of a 50.0 mL portion to a phenolphthalein end point requires 22.1 mL of 0.100 M HCI. A second 50.0-mL aliquot requires 48.4 mL of the HCI when titrated to a bromocresol green end point. 24. What is the composition of the sample? A. NaOH only B. Na2CO3 only C. NaOH and Na2CO3 D. Na₂CO3 and NaHCO3 nol 25. What are the concentrations of the components of the sample? A. 0.0084 M NaOH B. 0.0442 M Na2CO3 C. 0.0084 M NaOH and 0.0442 M Na2CO3 D. 0.0442 M Na2CO3 and 0.0084 M NaHCO3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY