
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
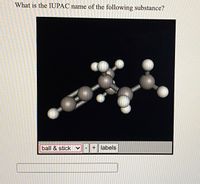
Transcribed Image Text:**Title: Determining the IUPAC Name of a Molecule Using a Ball and Stick Model**
**Question:**
What is the IUPAC name of the following substance?
**Description:**
The image displays a ball and stick model of a molecular structure. The model is set against a black background, highlighting the bonds and atoms clearly.
**Components:**
- The atoms are represented by spheres of different sizes and colors, indicating different elements.
- The bonds between atoms are represented by sticks, showing connectivity and bond angles.
- This 3D visualization helps in understanding the spatial arrangement of the atoms within the molecule.
**Interactive Features:**
- The display includes a dropdown menu labeled "ball & stick," suggesting that different visualization modes may be available.
- Two buttons marked "+" and "-" indicate options to zoom in or out.
- An option labeled "labels" suggests that users can toggle identifiers for each atom within the molecule.
This representation is a useful tool for learning about molecular geometry and nomenclature, helping students visualize and understand complex chemical structures.
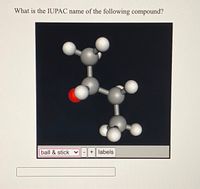
Transcribed Image Text:**Title:** Determining the IUPAC Name of Organic Compounds
**Content:**
In this educational module, we focus on identifying the IUPAC names of organic compounds using molecular models. Below is an example of a molecular structure represented using a ball-and-stick model, which helps visualize the spatial arrangement of atoms. Each ball represents an atom, and the sticks represent chemical bonds.
**Visual Analysis:**
The diagram displays a 3D ball-and-stick model of a compound. The model consists of:
- **Gray spheres:** Represent carbon atoms.
- **White spheres:** Represent hydrogen atoms.
- **Red sphere:** Represents an oxygen atom.
This particular setup suggests the presence of an alcohol functional group due to the presence of the red sphere (oxygen) bonded to a carbon (gray sphere).
**Understanding the Structure:**
1. **Main Carbon Chain:**
- The model contains three carbon atoms forming a chain.
2. **Functional Group:**
- The red sphere indicates an alcohol group (OH) attached to the second carbon atom in the chain.
3. **Hydrogen Atoms:**
- The white spheres show the hydrogen atoms completing the valency for each carbon atom.
By analyzing the structure, we deduce the compound is **propan-2-ol** (also known as isopropanol or isopropyl alcohol) because the OH group is attached to the second carbon of a three-carbon chain.
**Interactive Features:**
The interface allows users to switch between different molecular representations (e.g., ball & stick, space-filling), adjust the model size, and enable/disable atom labels for clarity.
This tool is instrumental for students learning organic chemistry, offering a hands-on approach to understanding molecular geometry and functional groups.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- * Question Completion Status: 10 40 60 100 A Moving to another question will save this respons Question 9 What is the IUPAC name of the following compound? Br Br CH3 CH3-CH2-CH-CH-CH2-0-CH-CH3 OA. propoxy-3,4-dibromo pentane O B. İsopropoxy-2,3-dibromo pentane c.5-propoxy-3,4-dibromo pentane D. 5-isopropoxy-3,4-dibromo pentane O E. 5-propoxy-3,4-bromo heptane A Moving to another question will save this response.arrow_forwardhow do you name this? thank you !!arrow_forwardNo line formula please 3-methyl-2-heptanol + H2SO4arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY